Scientists have identified a single molecule that may explain how bacteria can trigger one of the most severe types of asthma, a discovery that for the first time identifies the “missing link” between exposure to bacterial components and extreme inflammation of the lungs’ airways.
The new research not only clarifies how a severe form of asthma affects patients, but further underscores how bacterial dysbiosis—disruptions in beneficial bacteria amid exposure to pathogenic forms—affects vulnerable lungs. Going into the research, scientists already knew that bacterial molecules can trigger inflammatory activity in the lungs’ airways because patients with severe asthma often have changes in their bacterial populations. Yet the exact mechanisms by which bacteria exacerbate asthma remained unclear.
Seeking answers, Dr. Sarah Headland and colleagues in the immunology division of Genentech in south San Francisco, zeroed in on a form of asthma known as non-type-2 to find out why it is one of the severest forms of inflammatory respiratory disease. She and her team have also begun the arduous task of developing a customized therapy.
Writing in Science Translational Medicine, the team describes the research that allowed a better understanding of this form of asthma and the first steps toward a therapy geared specifically for patients with non-type-2 disease. They began by analyzing the cells and tissues from patients encumbered by severe bacteria-associated asthma and comparing those findings to the cells and tissues of people with mild to moderate forms of asthma as well as to those who don’t have asthma at all.
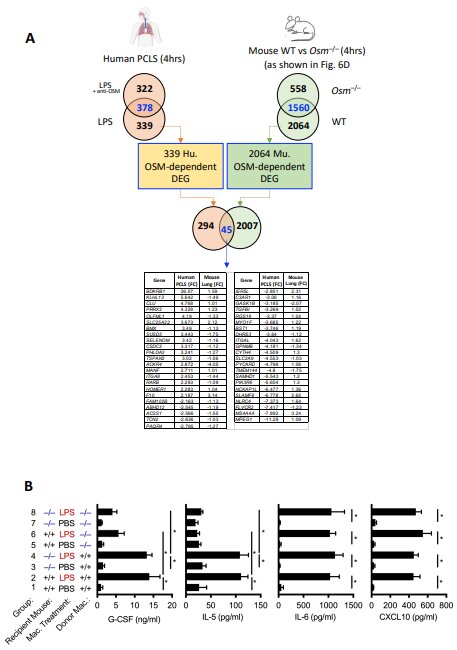
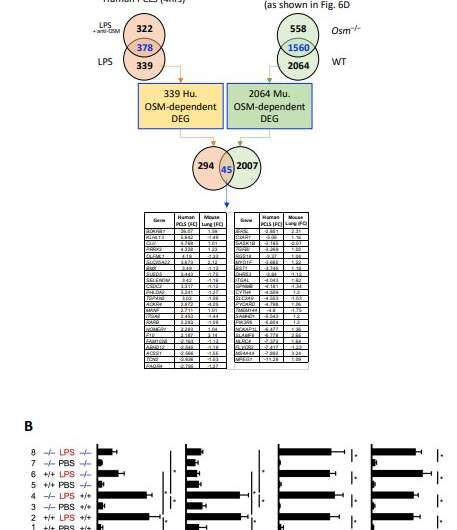
Headland and colleagues studied airway biopsies from 57 patients with severe asthma, 28 patients with mild or moderate asthma, and 16 healthy individuals. The key discovery was abnormally high activity of oncostatin M, a protein associated with inflammation and an aggressive immune response, that was unique among patients with severe asthma. Additionally, exposure to lipopolysacharride—LPS, a component of bacterial cell walls—triggered the activity of oncostatin M.
“Bacterial dysbiosis and opportunistic bacterial infections have been observed in, and may contribute to, more severe asthma,” wrote Headland in Science Translational Medicine. “However, the molecular mechanisms driving these exacerbations remain unclear. We show here that bacterial lipopolysaccharide induces oncostatin M and that airway biopsies from patients with severe asthma present with an OSM-driven transcriptional profile.
“This profile correlates with activation of inflammatory and mucus-producing pathways,” Headland added, noting that using “primary human lung tissue or human epithelial and mesenchymal cells, we demonstrate that oncostatin M is necessary and sufficient to drive pathophysiological features observed in severe asthma after exposure to LPS.”
While the new analysis helped scientists gain a keener understanding of the underlying drivers of severe bacteria-associated asthma, there were also suggestions in their research that a monoclonal antibody may one day block oncostatin M. Both lines of research—discovering the missing link in severe bacteria-associated asthma and pinpointing a potential form of treatment—provide a ray of hope for patients with the form of the disease broadly known as non-type-2.
Asthma was once thought to be a single disorder, but doctors now understand it to be several complex but related conditions with varying underlying triggers. There are two key categories of severe asthma: Type 2 inflammation and non-type 2 inflammation. Each of the two categories is based on the biological mechanisms that drive the disease. Type 2 inflammation, for example, includes allergic asthma and eosinophilic asthma.
The Asthma and Allergy Foundation of America defines allergic asthma as an inflammatory disease caused by an allergen, such as exposure to cockroaches, pollen, dust mites, mold or pet dander, to name a few triggers. The immune system responds by producing an overabundance of the immunoglobulin (antibody) known as Immunoglobulin E, or IgE. Extremely high levels of IgE can cause inflammation of the lungs’ airways.
Another form of Type 2 is eosinophilic asthma, which is characterized by high levels of white blood cells known as eosinophils. A hallmark of this type of asthma is generalized swelling throughout the entire respiratory tract, from the nasal region to the tiniest airways in the lungs. People with this form of asthma experience wheezing, shortness of breath, chest tightness and lung-function abnormalities, among other symptoms.
Non-type 2 inflammation in severe asthma has been defined as the absence of eosinophils. However, doctors say there is much more to this form of asthma, which is characterized by a constellation of problems, ranging from an extreme inflammatory condition in the lungs airways to shortness of breath and difficulty controlling the condition. In terms of treatment, medical experts also have long known that non-type 2 inflammation doesn’t respond to inhaled corticosteroids, a standard of care that works well in other forms of asthma. Thus, the discovery of a bacterial dysbiosis associated with oncostatin M, opens a new window of understanding into a debilitating form of the disease, Headland and colleagues reported.
Indeed, there is a glaring unmet need for patients with this form of asthma because a specific therapy—something targeted—to address the unique manifestations of this form doesn’t exist.
Headland and her collaborators found that oncostatin M drives the core asthma features, such as inflammatory signaling and excessive mucus production, when exposed either to LPS or a common bacterial pathogen, Klebsiella pneumoniae. And because of the unique role played by oncostatin M, the Genentech scientists are developing a potent monoclonal antibody that can block the protein, staving off airway inflammation.
Source: Read Full Article