The choice of microplate for an assay depends on a variety of factors, but it all starts with the requirements of the result expected from the assaying process, and the rest is worked out backward. The diagram below is a brief summary of what happens when the decision-making process is undertaken:
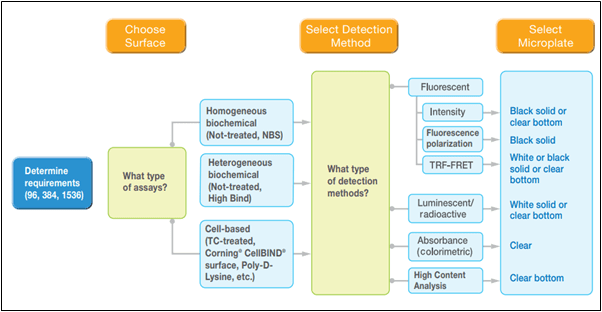
Figure 1. The microplate selection process. (image credit: https://www.corning.com)
The image describes three broad stages through which a scientist must think before arriving at the right testing equipment for the planned experiments. In lab tests, it is critical that the right equipment is used (the components are as important as the configuration of the components used in the process).
For example, using the 96 well plates might be crucial in a process where the 384 well plates would not produce accurate results. Further, the 96 well plates might need a two wall polymer profile for a lipid profiling reaction, which means using a single polymer profile would be detrimental to the results. This means the failure of the test must be isolated from the failure of the right environment for conducting the tests, and equipment forms a major part of this environment.
The decision-making process
The first step in the decision-making process is the selection of the microplate material and color required for running the tests. The options for the material are polystyrene, polypropylene, cyclo-olefins, vinyl, and UV transparent materials. Then depending on the speed and nature of the reactions, the next step is deciding the number of wells in the micro-plate.
 Image Credits: Choksawatdikorn / Shutterstock.com
Image Credits: Choksawatdikorn / Shutterstock.com
The options available are 6 wells, 12 wells, 24 wells, 48 wells, 96 wells (and 96 strip wells and half wells), 384 wells and 1536 wells (though 3456 wells and 9600 wells have been made, they remain a rare commodity). The number is directly linked to the volume to be handled.
On the one hand are the 96-well plates, which handle about 320 to 360 μL per well, and on the other, there are 1536-well plates, which handle anywhere between 10.7 and 12.8 μL typically. Similarly, the working volume in the wells also varies (it being 50 μL to 200 μL for the 96 well plates, and 1 μL to 10 μL for the 1536 well plates).
Once the well number has been decided, the next step is to choose the type of assay to be used, on the basis of the adhesion and biochemical requirements of the test. The type of treatment and the cell-binding nature decides the level of cell attachment, the type of anchorage and the growth rate of dependent cells, making the assay selection process crucial to the progress of the reaction.
The options available are high binding enzyme type, non-binding surface type, and ultra-low attachment surfaces. Colorimetric assays can be used for absorbance testing, luminometric assays are used for luminescence testing, fluorometric assays are used for fluorescence testing, and special assays are dedicated for high content analysis.
Once the size of the cell array and the surface properties have been decided, the next step is deciding the color of the wells, depending on the fluorescence and luminescence properties required. Black color reduces the light scattering and results in higher signal to noise ratios, while white enhances luminescence signal to noise ratio by reflecting light back into the detector.
The shape of the well bottoms is also important, and the number of wells is also linked to the shape being chosen. V-bottom, conical, and easy wash bottoms are available only in 96-well plates, while flat and round bottoms are available in other sizes (such as 384 well and 1536 well plates).
Finally, in multi-well microplates, the type of molding is also important in deciding the final matrix structure between the wells in a microplate. Thermally bonded polymer wells are produced by molding two different kinds of polymers and allowing them to form a walled structure so that the base, sidewalls, or the top film are made of precise compositions, as required by the reaction process. Hence, the injection molding is done in a specific manner to ensure that the two or multiple well systems work fine.
Finally, the microplate must be accompanied by support instrumentation, which complements the placement, transfer, and extraction of substrates in and out of the plate wells. The choice of the instrumentation also depends on the assay to be used, and the readers, sealers, washers, movers, and insulators are all decided basis the assays being used and the well configuration.
Conclusion
As can be expected, the decision-making process for choosing the right microplate for an assay is a complex one. There are a host of parameters to be accounted for, and it is common for researchers to realize that the biochemical reactions did not produce the desired results because the testing equipment was not used properly.
In this respect, the above step by step articulation of the process lays out a structured process to find the right equipment to be used for the medical testing procedures.
Sources
Corning (2015), Corning and Falcon micro-plates selection guide, https://www.news-medical.net/health/Is-Red-Wine-Good-for-the-Body.aspx
Wellplate (2019), Select the right well plate material for highest quality for reproducible results from test to test! https://www.wellplate.com/well-plate-material/
Further Reading
- All Microplate Content
- The History of Microplates
- What are Microplates?
Last Updated: Jan 7, 2021
Source: Read Full Article
