The protein behind immunotherapy resistance
Immunotherapy is a cutting-edge approach to treating cancer by turning the patient’s own immune system against their tumor. Our increasing knowledge of the mechanisms by which the body regulates immune responses has been transformative to our fight against cancer.
But despite success rates, immunotherapy has time and again met with a stubborn obstacle: tumor cells often evade the “radar” of immune cells seeking to destroy them. This in turn leads to treatment resistance, which in many cases would benefit from a deeper understanding of mechanisms that can help circumvent it.
A new study led by scientists at EPFL has now uncovered a protein that plays a key role in helping tumors evade immune destruction. The protein, named “fragile X mental retardation protein” (FMRP), regulates a network of genes and cells in the tumor microenvironment that contribute to its ability to “hide” from immune cells. Normally, FMRP is involved in regulating protein translation and the stability of mRNA in neurons. But the researchers found that it is aberrantly up-regulated in multiple forms of cancer.
The study, published in Science, was led by researchers in the group of Douglas Hanahan at the Swiss Institute for Experimental Cancer Research (ISREC) and the Lausanne Branch of the Ludwig Institute for Cancer Research, along with colleagues from the University Hospital of Lausanne (CHUV) and other Swiss institutions. The discovery has also led to an EPFL spin-off, Opna Bio, whose staff were also involved in the research.
But why FMRP? The idea came from previous studies showing that cancer cells that naturally overexpress FMRP are invasive and metastatic. Other studies show that if, in contrast, FMRP fails to be expressed in developing neurons it can lead to cognitive defects (hence the “mental retardation” part of the protein’s name).
With this evidence in mind, the researchers set off to investigate the expression of FMRP in human tumors. They then assessed its tumor-promoting functions in mouse models of cancer, and finally studied its association with prognosis for human cancer patients.
The study involved several data-gathering steps. First, the scientists performed immunostaining for FMRP on tissue from human tumors. The majority of the tumors tested positive, while corresponding normal tissue did not. This meant that FMRP is specifically and highly expressed in cancer cells.
The team then moved onto the main part of their research, which was to determine the functional significance of FMRP in those tumors –they express the protein, but what does it do?
FMRP is involved with the immune system
To explore this, the scientists developed lines of so-called “knockout” cancer cells. Knockout cells or organisms are genetically engineered to lose—”knock out”—a specific gene in order to find clues about its function. Essentially, whatever change occurs in knockout cells compared to cells that still have the gene—called “wild-type”– can generally be traced back to the missing gene.
In this case, the scientists used the famous CRISPR-Cas9 gene-editing technique to knock out the gene (called FMR1) that produces FMRP in mouse cancer cells arising from pancreas, colon, breast, and skin melanocytes. They then compared the FMRP-knockout cancer cells to cancer cells that still had the FMR1 gene and thus expressed the FMRP protein.
The researchers evaluated survival rates between mice with tumors containing FMRP-knockout cancer cells and those with FMRP-wild-type cells, first in mice whose immune systems had been compromised. The comparison revealed similar survival rates. In notable contrast, when they compared the knockout tumors to wild-type tumors growing in mice with properly functioning immune systems, they found that tumors without FMRP were growing slower, and the animals survived longer.
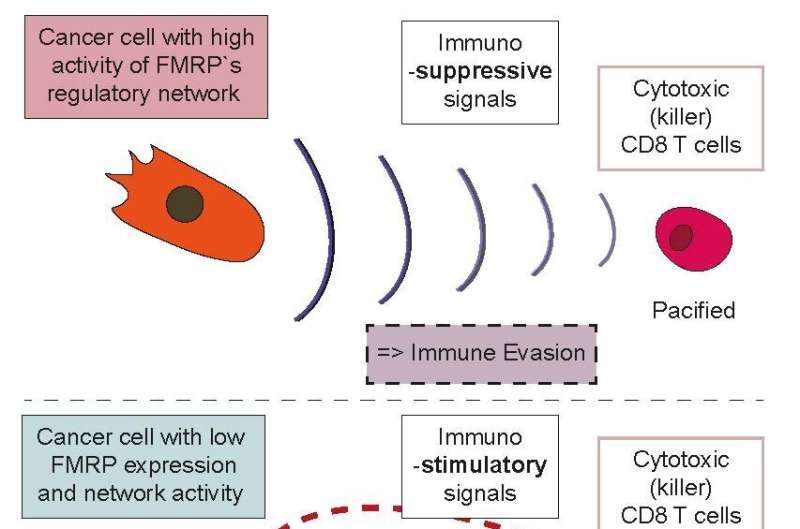
What this part of the study showed was that that FMRP is not involved in stimulating tumor growth per se, and rather implicated the adaptive immune system (the part of our immune system that we “train” with vaccines).
This was further confirmed by the observation that wild-type tumors had very few infiltrating T lymphocytes, whereas knockout tumors were highly inflamed. Depleting T cells from the FMRP-knockout tumors caused them to start growing more rapidly and reduced the survival rates of the mice, meaning that FMRP is somehow involved in tumors evading the immune system.
How tumors with FMRP defend against immune cells
The team continued with molecular genetic profiling of both knockout and wild-type tumors. This revealed significant differences in gene transcription across the entire genome, suggesting that FMRP interacts with multiple genes. In addition, the tumors showed marked differences in the abundance of cancer cells, macrophages, and T cells, further implicating the role of FMRP in modulating components of the immune system.
The next phase of the study looked at the production of specific factors associated with the distinctive immune responses—evasion vs. attack. The tumors expressing FMRP were found to produce interleukin-33, a protein that induces the production of regulatory T cells, a specialized subpopulation of T cells that inhibit immune responses.
They also produce protein S, a glycoprotein that is known to promote tumor growth. Finally, the tumors produce exosomes—cell organelles that proved to trigger the production of a type of macrophage cell that normally helps with wound healing and tissue repair. Collectively, all three factors are immunosuppressive and contribute to the tumor’s barrier against attacks from T lymphocytes.
In contrast, the FMRP knockout tumors cells actually downregulated all three factors (interleukin-33, protein S, and exosomes) while they up-regulated a different chemokine called “C-C motif chemokine ligand 7” (CCL7), which helps recruit and activate T cells. This process is further aided by inducing immunostimulatory (and not immunosuppressive) macrophages. These cells produce three other proinflammatory proteins that work with CCL7 in recruiting T cells.
Predicting immunotherapy outcomes in human patients
In a clinical context, the question is whether levels of FMRP can help form a prognosis for patients undergoing immunotherapy. Counterintuitively, both mRNA of the FMR1 gene and FMRP protein levels were insufficient for predicting outcomes in cohorts of cancer patients.
To address this, the researchers built on the fact that, in the cell, FMRP up- and down-modulates the stability of mRNA by binding it directly. This means that FMRP might change RNA levels that could be picked up in transcriptome datasets, which could be collected to define a “gene signature” to help track its functional activity. The approach worked, allowing the scientists to track a gene signature of FMRP’s cancer regulatory activity with a network of 156 genes.
The FMRP cancer network activity signature proved to be prognostic for poor survival across multiple human cancers, consistent with the immunosuppressive effects of FMRP, and, in some patients, it was linked to poor responses to immunotherapy treatments.
The work shows that FMRP regulates a network of genes and cells in the tumor microenvironment, all of which help tumors to evade immune destruction.
Douglas Hanahan says, “Having studied the complex cellular composition of solid tumors for decades, I am personally astonished by our discovery that a co-opted neuronal regulatory protein—FMRP—can orchestrate the formation of a multi-faceted protective barrier against attack by the immune system that consequently limits the benefit of immunotherapies, thereby presenting FMRP as a new therapeutic target for cancer.”
More information:
Qiqun Zeng et al, Aberrant hyperexpression of the RNA binding protein FMRP in tumors mediates immune evasion, Science (2022). DOI: 10.1126/science.abl7207. www.science.org/doi/10.1126/science.abl7207
Journal information:
Science
Source: Read Full Article



