The fast track to a ‘Fortitude Kit’ for rapid COVID-19 diagnosis
It may seem like a lifetime ago, but it was only in late January 2020 that Singapore confirmed its first case of the then-novel coronavirus causing COVID-19. Amid the frenzied atmosphere of the Chinese New Year holidays, few would have suspected that the strange pneumonia would snowball into the global pandemic that it is today.
But a couple of scientists at Singapore’s Agency for Science, Technology and Research (A*STAR) had their ears pricked up. “We already heard through different channels in December that there was some sort of pneumonia in Wuhan,” recalled Dr. Sebastian Maurer-Stroh, Deputy Executive Director (Research) of A*STAR’s Bioinformatics Institute (BII).
Haunted by the memory of previous outbreaks—including SARS in 2003, swine flu in 2009 and Zika in 2016—local researchers jumped into action even before the virus had reached local shores. With the advancement of sequencing technologies over the years, the draft genome of the SARS-CoV-2 virus was released by Chinese researchers as early as mid-January, allowing scientists worldwide to quickly create their diagnostic kits.
By the first week of February, A*STAR researchers, along with their collaborators at Tan Tock Seng Hospital (TTSH), had already unveiled a locally developed COVID-19 diagnostic, called the Fortitude Kit, and sent it to various local hospitals, both local and overseas. In stark contrast, during the 2003 SARS outbreak, diagnostic kits were made available in Singapore only months into the outbreak. Here’s a look into the Fortitude kit’s fast track journey from bench to bedside.
Spotting a stealthy killer
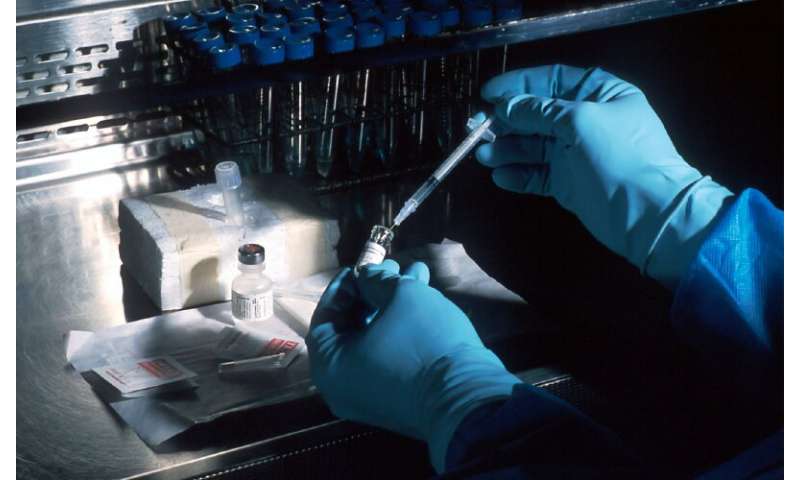
The Fortitude Kit is based on a technique known as the real-time reverse transcription polymerase chain reaction (RT-PCR). The testing process begins by collecting nasal or nasopharyngeal swabs from individuals suspected to have COVID-19. Once transported to the laboratory, viral RNA is extracted from these swabs. That is where the Fortitude Kit itself comes into the equation.
Each kit comprises a one-step RT-PCR test, which comes ready-made with all the reagents needed in the correct amounts. These reagents include reverse transcriptase, an enzyme that converts viral RNA to DNA, as well as short DNA sequences called primers that can detect SARS-CoV-2’s unique genetic footprint. During RT-PCR, primers recognize and bind to the viral DNA sequences converted from RNA. Repeated cycles of heating and cooling then trigger the Taq polymerase to exponentially create identical copies of viral DNA until they are detected by the machine.
Running the Fortitude Kit only takes around 90 minutes, but the preparation steps add on a few hours and it can take a day or longer for results to be released, depending on the resources of the healthcare providers.
Still, RT-PCR is widely considered to be the gold standard for the detection of viruses such as SARS-CoV-2. Unlike other diagnostics on the market, RT-PCR-based tests have specificity rates of over 99 percent—meaning that individuals who test positive truly have the disease. The sensitivity of RT-PCR tests depends on the stage of illness, being close to 100 percent in the first week of illness but then becoming less sensitive as time goes by. So far, Fortitude has become one of the most widely adopted diagnostic kits on the market.
All hands on deck
The Fortitude Kit was made possible by a powerhouse team of Singapore’s leading scientific minds, namely A*STAR scientists, Dr. Maurer-Stroh and Dr. Masafumi Inoue; as well as CEO of the Diagnostics Development (DxD) Hub Dr. Sidney Yee, and TTSH’s Dr. Timothy Barkham. Each expert brought their own specialized set of skills to the table in the fight against COVID-19.
With his expertise in computational biology, Maurer-Stroh examined the virus’ genetic sequences and its evolution over time, sharing his insights on the Global Initiative on Sharing All Influenza Data (GISAID) public database. Armed with this data, Inoue, Head of the Diagnostics Group at A*STAR’s Experimental Drug Development Centre (EDDC), then started developing the prototype of Fortitude Kit. The first order of business? Designing primers that would target SARS-CoV-2.
“Ensuring quality primer designs is always our focus in developing diagnostic kits,” said Inoue. To do so, his team first had to identify a distinct sequence to target that was also shared by the current outbreak strains. This required Inoue to compare and contrast multiple coronavirus genomes, enlisting help from colleagues like Maurer-Stroh to narrow down the choice of regions to target using bioinformatics. In the end, they went for a region which codes for an enzyme that catalyzes the replication of RNA from an RNA template.
“We chose that region as a primer target since we knew it had the least number of mutations in the 2003 SARS virus,” explained Inoue. By designing primers based on the unique portions of the virus that are less prone to mutations, more circulating viral strains can be readily detected by the kit.
Once the primers and the prototype were in place, TTSH’s Barkham stepped in to fine-tune the kit’s parameters. His team tweaked the RT-PCR process at various points, carrying it out at different concentrations or temperatures to identify the ideal conditions. They also evaluated the kit on real patient samples, which could contain molecules that may confound the test and result in inaccurate readings.
Fortitude’s finishing touches were then added by the Yee’s team at DxD Hub, a national platform led by A*ccelerate, A*STAR’s commercialization arm. “We optimized the assay so that we are sure that it works every single time,” she shared. “We also developed the whole production process, because it’s more than just putting things together in a kit. It’s also a lot of manufacturing instructions, quality control and quality assurance protocols.”
Given the massive effort required to develop a diagnostic, it’s remarkable that A*STAR and TTSH managed to successfully do so in less than a month. Their long, sleepless nights were powered by the sheer willpower to help Singapore face its biggest crisis yet. “This is only a small snapshot of the team involved in rolling out just one product, Fortitude Kit,” commented Yee. “It really takes the whole Singapore R&D ecosystem and we’re very fortunate to be part of it.”
Singapore’s gift to the world
In Singapore, the Fortitude Kit has been in routine use in 13 public and private hospitals and laboratories since February. To date, the kit has also been deployed in more than 20 countries. While the DxD Hub produced the initial batch of kits, the know-how was transferred non-exclusively to a handful of biotech companies to scale-up and manufacture the diagnostic tests.
From a weekly output of 100,000 tests at the start of the outbreak, the manufacturing transfer exercise has ramped up production to over four times that per week. “Fortitude is now in around 20 countries. It is probably one of the most widely used RT-PCR test kits in the world,” shared Yee. Beyond its obvious clinical benefit, Fortitude Kit has also helped spread the branding of Singapore and A*STAR worldwide, she added.
Still, the work continues. “RNA viruses are mutating all the time,” explained Maurer-Stroh. Most mutations are tiny changes, but closely monitoring the evolution of the virus allows A*STAR to quickly respond and modify Fortitude if needed.
Source: Read Full Article


