Study: The human body may possess a secret weapon against SARS-CoV-2
An obscure class of molecules, part of the vast system that helps the human body distinguish “self” from “non-self,” may also hold the key to stopping SARS-CoV-2 from commandeering healthy cells, scientists have found in an elegant series of experiments.
The research, published in Science Immunology, was led by the University of Oxford in the U.K., where scientists have homed in on a class of molecules whose job is preventing the immune system from attacking healthy cells. The spotlighted molecules belong to the human leukocyte antigen class of proteins, part of the major histocompatibility complex. The human leukocyte antigen proteins are known by the shorthand description HLA.
Within the crucial HLA family, there are several classes of HLA molecules, and each class can be found on specific types of cells. For example, HLA class I molecules are found on the surface of almost all nucleated cells; class II are on B lymphocytes, dendritic cells, macrophages, monocytes, and thymic epithelial cells, among others. These HLA groups, or classes of HLA molecules, are known as classical HLA proteins.
It is HLA antigens involving donors and recipients that are analyzed for organ transplants. The closer the match, the more likely that the transplant will be successful. HLA proteins, in general, enable the body to distinguish “self” from “non-self,” and prevent autoimmune attacks against normal cells.
Even though past research has demonstrated that SARS-CoV-2 infection automatically tamps down—downregulates—classical HLA proteins to prevent the immune system from recognizing infected cells, it has remained unclear whether SARS-CoV-2 truly affects all HLA classes in the same way. For example, the behavior of an obscure subset of HLA class I molecules, defined as HLA-E, is not tamped down by the virus the way classical HLA molecules are, suggesting a possible covert immune response against SARS-CoV-2.
HLA-E is expressed on natural killer cells and on CD8+ T cells. The Oxford team was interested in the population known as HLA-E-restricted CD8+ T cells, a fierce but clandestine army of T cells potentially capable of quashing cells infected with SARS-CoV-2. It’s the activity of these T cells that may explain why some people quickly thwart COVID-19.
Still, as exciting as the possibility of a secret weapon against the coronavirus seemed in theory, the activity of HLA-E during SARS-CoV-2 infection had been murky until the new Oxford research.
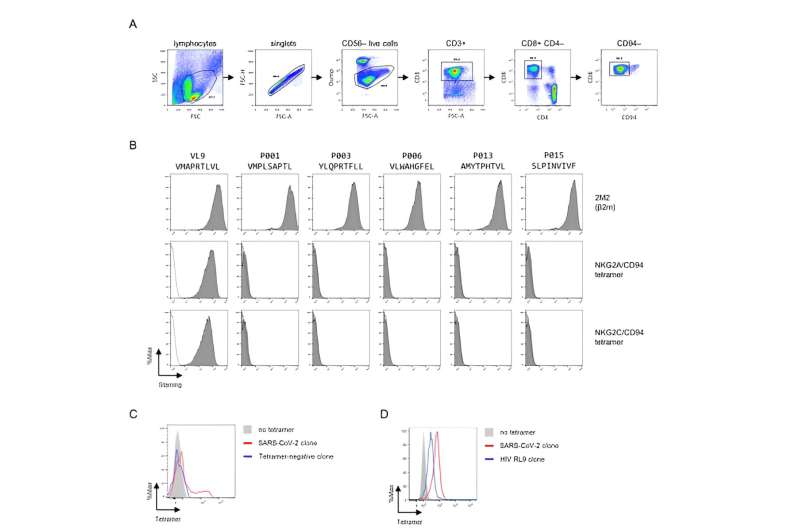
Dr. Hongbing Yang and a team of Oxford colleagues have examined blood samples from patients with COVID-19 and identified five peptides—protein fragments—that induce HLA-E-restricted killer T cell activity. HLA-E is an important regulator of natural killer cell and cytotoxic T lymphocyte activation and inhibition. HLA-E, the team found in a series of experiments, can respond to and initiate an immune response against SARS-CoV-2-infected cells.
“We describe five peptides from SARS-CoV-2 that elicited HLA-E–restricted CD8+ T cell responses in convalescent patients with coronavirus disease 2019,” wrote Dr. Hongbing Yang in the research paper, referring to patients with COVID-19.
Yang and his Oxford team demonstrated in their research how HLA-E is critical during SARS-CoV-2 infection. Indeed, the HLA system is controlled by genes on chromosome 6, which encode the activity of all HLA cell-surface proteins that help regulate the immune response. The Oxford team zeroed in on HLA-E because it is known for its capacity to orchestrate the destruction of cells compromised by replicating viruses.
In the laboratory, Yang and colleagues discovered that clones of the HLA-E-specific CD8+ T cells, derived from COVID-19 patients, recognized SARS-CoV-2–infected cells and suppressed viral replication. But, also confirmed in the laboratory was the tamping down of classical HLA molecules by SARS-CoV-2. This is the activity that allows the coronavirus to overwhelm vulnerable cells while eluding the immune system—the so-called immune escape. But this did not happen in HLA-E cell lines, particularly those from primary human airway epithelial cells, the study found.
Further analyses of primary human lung epithelial cells showed that SARS-CoV-2 exposure caused downregulation of HLA-Ia, but not HLA-E. HLA-E peptide-derived CD8+ T cell clones, which expressed diverse T cell receptors, and suppressed SARS-CoV-2 replication in human lung epithelial cells. “It is possible that the normal balance, which is strongly in favor of classically restricted T cells, is disturbed in SARS-CoV-2 infection and that HLA-E-restricted T cells then could contribute to control of the virus in this infection,” Yang and colleagues theorized in the study.
Their research has opened a new window of understanding into the HLA complex in general during SARS-CoV-2 infection, and more specifically, HLA-E’s unique response. Yang and collaborators discovered that HLA-E-restricted killer T cells persisted at a robust level, and that the HLA-E T cells were capable of suppressing viral replication.
These findings suggest that SARS-CoV-2–specific HLA-E–restricted CD8+ T cells may be advantageous when classical HLA molecules are downregulated by SARS-CoV-2. Increasing the activity of HLA-E-restricted molecules could be exploited through a targeted vaccine, especially for people who are immunocompromised, such as people 65 and older, or those who have chronic diseases.
“Induction of HLA-E-restricted T cell responses by vaccines focused on the universally presented epitopes could be an attractive possibility,” Yang concluded.
More information:
Hongbing Yang et al, HLA-E–restricted SARS-CoV-2–specific T cells from convalescent COVID-19 patients suppress virus replication despite HLA class Ia down-regulation, Science Immunology (2023). DOI: 10.1126/sciimmunol.abl8881
Journal information:
Science Immunology
Source: Read Full Article



