Immunology research led by Harvard Medical School has taken a closer look at the role of B-cells in fighting cancer. In a paper, “B-cell-specific checkpoint molecules that regulate anti-tumor immunity,” published in Nature, the team identifies a critical checkpoint of B-cell activation and how bypassing the checkpoint could unlock T-cell potential.
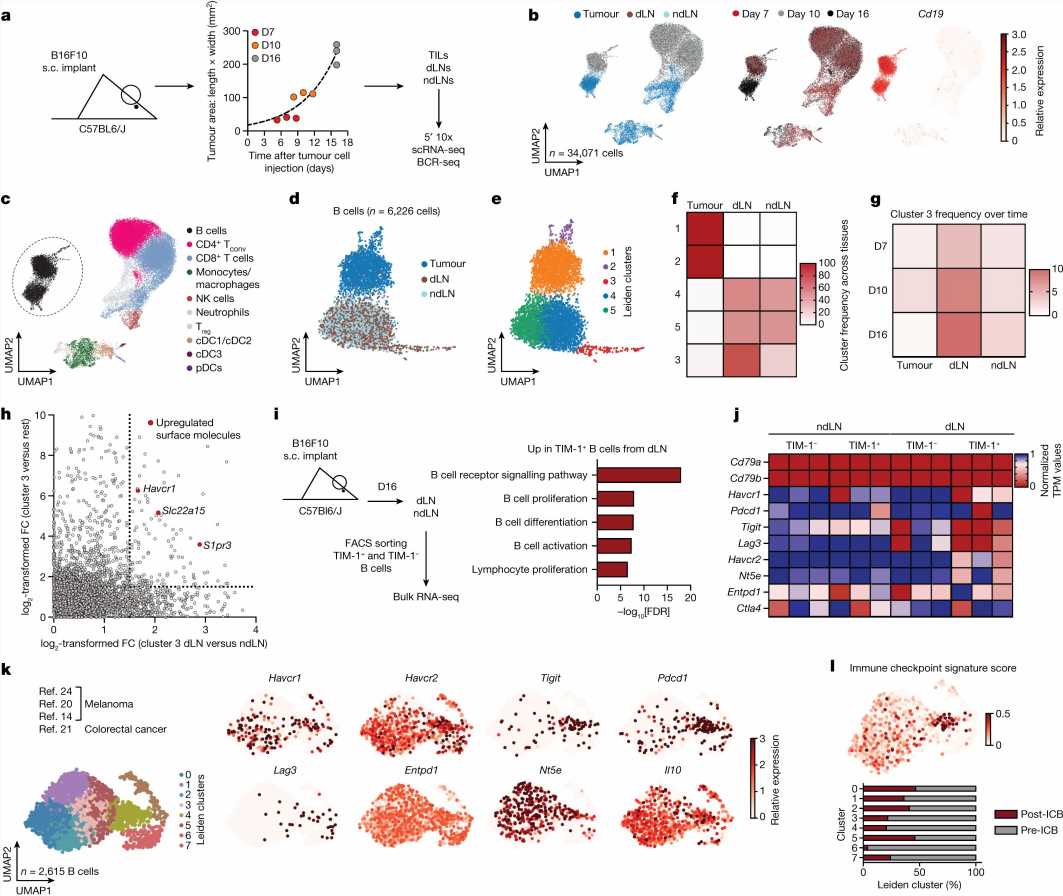
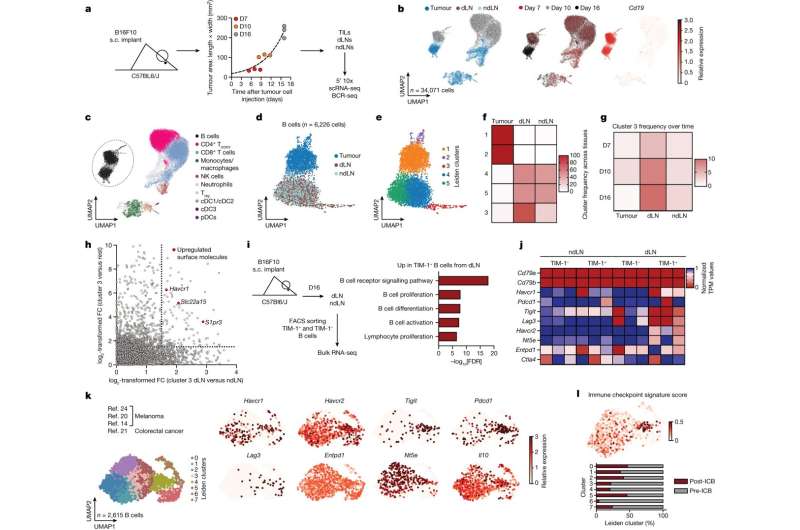
Researchers used high-throughput flow cytometry, bulk and single-cell RNA-sequencing to study various B-cell types during melanoma growth and monitored gene expression levels.
They found that the depletion of B-cells globally significantly enhanced melanoma tumor growth.
A subset of B-cells that specifically expands in the draining lymph node in tumor-bearing mice was identified. This expanding B-cell subset expresses the cell surface molecule TIM-1, encoded by the gene Havcr1. The subset also expresses co-inhibitory molecules such as PD-1, TIM-3, TIGIT, and LAG-3.
Conditional deletion of the co-inhibitory molecules on B-cells had little or no effect on tumor burden. However, selective deletion of Havcr1 in B-cells significantly inhibited tumor growth.
With the loss of Havcr1 in knock-out mice, the accompanying absence of TIM-1 led to increased B-cell activation and antigen presentation resulting in the expansion of tumor-specific T-cell activity.
Administration of a commercially available high-affinity anti-TIM-1 antibody also inhibited tumor growth. Removal of the Havcr1 gene from T-cells had no effect, isolating the effect as B-cell dependent.
Specific B-cell tumor-related mechanisms are poorly understood in comparison to T-cells. More generally, it is a collaborative effort through a cascade of intracellular signaling to mount an effective immune response against pathogens. Within this system, there are signals that both initiate and regulate immune response activities.
This specific B-cell subset expression of TIM-1 may function as a healthy regulatory check on T-cell activation, suppressing excessive immune system response. That healthy function could be unconnected to tumor growth, putting it at odds with the ability of the immune system to mount a more active attack on tumor cells.
B-cell responses have previously been associated with positive outcomes in multiple cancers by fostering intratumoral B-cell and T-cell cooperation. The data in this study suggest that TIM-1 can causally limit B-cell activation, antigen presentation and T-cell activation.
Manipulation or suppression of TIM-1-expressing B-cells could have a therapeutic advantage in allowing the immune system to mobilize anti-tumor immunity and inhibit tumor growth.
More information:
Lloyd Bod et al, B-cell-specific checkpoint molecules that regulate anti-tumour immunity, Nature (2023). DOI: 10.1038/s41586-023-06231-0
Uncovering a role for B cells in antitumour immunity, Nature (2023). DOI: 10.1038/d41586-023-01678-7
Journal information:
Nature
Source: Read Full Article