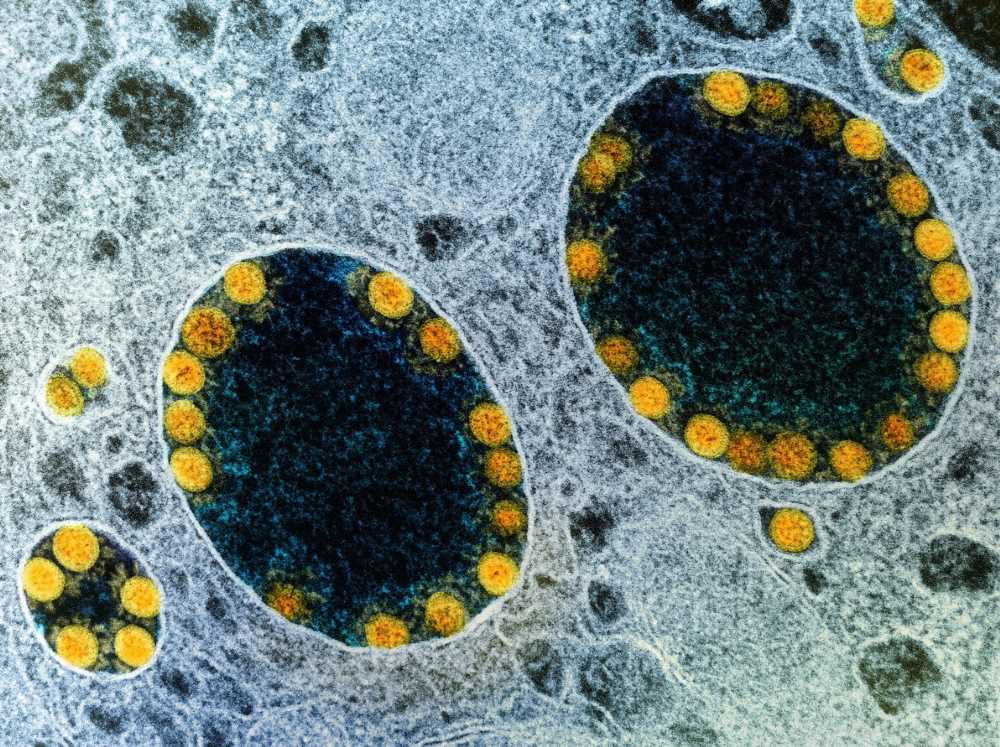
In a recent study published in the journal Nature Cell Biology, researchers demonstrated the susceptibility of salivary glands to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in vitro using an organoid culture model.
 Study: Human induced pluripotent stem cell-derived salivary gland organoids model SARS-CoV-2 infection and replication. Image Credit: NIAID
Study: Human induced pluripotent stem cell-derived salivary gland organoids model SARS-CoV-2 infection and replication. Image Credit: NIAID
Background
Genetics & Genomics eBook

Although the respiratory system, including the upper respiratory tract (URT) and lungs, are the main target sites of SARS-CoV-2, there is growing evidence that it affects multiple organs. For example, oral mucosa and salivary glands widely express two SARS-CoV-2 entry factors, angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2). Also, they serve as reservoirs of SARS-CoV-2.
Intriguingly, 80% of SARS-CoV-2 infections are asymptomatic. In addition, studies have reported that SARS-CoV-2 target salivary glands in humans and infectious virions from the saliva of asymptomatic individuals contribute to onward viral transmission.
Due to SARS-CoV-2’s ability to infect multiple types of organoids, an organoid culture model could prove apt to demonstrate the infection by SARS-CoV-2 in salivary glands (in vitro). However, due to a lack of appropriate in vitro models, studies have not been able to elucidate mechanisms underlying SARS-CoV-2 infection and replication in salivary glands and subsequent secretion into saliva.
About the study
In the present study, researchers generated functional salivary gland organoids from human induced pluripotent stem cells (hiPSCs) with similar morphological features and physiological functions (in vivo) as human salivary glands. In addition, these organoids had salivary gland-specific cell lineages.
The team performed single-cell ribonucleic acid sequencing (scRNA-seq) of hiSG cells on day 80 using the 10X Genomics platform. Further, they isolated the organoids on day 60 and cultured them with the fibroblast growth factor (FGF) 7 and 10 until day 80 to characterize the observed branching structures.
SRY-box transcription factor 9 (SOX9) gene regulates the development of both mouse and human salivary glands. In its absence, the salivary glands cannot undergo branching morphogenesis. Therefore, the researchers suppressed SOX9 during hiSG induction to elucidate whether hiSGs recapitulated the SOX9-mediated developmental process.
Study findings
The human-induced salivary glands (hiSGs) had properties similar to those of embryonic salivary glands, including morphological, protein marker expression, and gene expression characteristics. Consistent with in vivo studies conducted in mice, SOX9 knockdown inhibited branch formation in hiPSCs, suggesting that this gene is crucial for human salivary gland development. Thus, this study model could replace animal models used to study human salivary gland development.
The hiSGs morphologically and functionally mimicked natural salivary glands. Unbiased scRNA-seq clustering identified six major cell groups, further divisible into cellular subtypes. The small mesenchymal population in the HiSGs did not show features of salivary gland mesenchyme. Conversely, the epithelial cell group had five cell groups, acinar, basal, ductal, myoepithelial, and actively cycling cells. The hiSGs comprised independent lineages of basal–myoepithelial cells from the ductal–acinar lineage, and pseudotime analysis showed the differences in these two cell lineages.
The fundamental function of salivary glands is to secrete fluid induced by acetylcholine through muscarinic acetylcholine receptor pathways. In the hiSGs, treatment with carbachol, a muscarinic acetylcholine receptor agonist, transiently increased the intracellular calcium levels in a dose-dependent manner. Furthermore, orthotopically transplanted hiSGs presented the phenotype of mature salivary glands over time engrafted at a recipient site in mice.
The scRNA-seq and immunofluorescence analyses also revealed that the ductal cells within hiSGs abundantly expressed ACE2 and TMPRSS2, similar to normal salivary glands. The immunostaining method revealed that while apical side ductal cells of hiSGs strongly expressed ACE2, ductal cells and acinar cells expressed TMPRSS2. When exposed to SARS-CoV-2, hiSGs got infected and showed SARS-CoV-2 replication within 24 hours post-infection. The authors also detected the SARS-CoV-2 nucleoprotein in infected ductal cells. Accordingly, the median tissue culture infectious dose (TCID50) assay showed infectious viruses in hiSGs, which peaked at 24 hours but declined later.
Conclusions
Indeed, the hiSGs developed in the current study could serve as a valuable model for reproducing heterogeneous cell populations in human salivary glands. Furthermore, hiSGs could aid the functional analysis of genes during development and serve as a promising tool for investigating SARS-CoV-2 infection in salivary glands at the molecular level. Furthermore, the researchers showed that hiSGs had several advantages compared to organoids derived from adult human salivary gland-derived tissue stem progenitor cells. For example, they could be easily genetically modified using clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein 9 (Cas9) gene-editing tool and were relatively easy to culture.
- Tanaka, J., Senpuku, H., Ogawa, M. et al. Human induced pluripotent stem cell-derived salivary gland organoids model SARS-CoV-2 infection and replication. Nat Cell Biol (2022). https://doi.org/10.1038/s41556-022-01007-6, https://www.nature.com/articles/s41556-022-01007-6#Sec7
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: ACE2, Agonist, Angiotensin, Angiotensin-Converting Enzyme 2, Assay, Calcium, Cas9, Cell, Cell Biology, Coronavirus, Coronavirus Disease COVID-19, CRISPR, Cycling, Enzyme, Fibroblast, Gene, Gene Expression, Gene-Editing, Genes, Genomics, Growth Factor, in vitro, in vivo, Induced Pluripotent Stem Cells, Intracellular, Lungs, Mesenchyme, Organoids, Palindromic Repeats, Phenotype, Progenitor Cells, Protein, Receptor, Respiratory, Ribonucleic Acid, Salivary Gland, SARS, SARS-CoV-2, Serine, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Stem Cells, Syndrome, Tissue Culture, Transcription

Written by
Neha Mathur
Neha is a digital marketing professional based in Gurugram, India. She has a Master’s degree from the University of Rajasthan with a specialization in Biotechnology in 2008. She has experience in pre-clinical research as part of her research project in The Department of Toxicology at the prestigious Central Drug Research Institute (CDRI), Lucknow, India. She also holds a certification in C++ programming.
Source: Read Full Article