New combination of drugs works together to reduce lung tumors in mice
Cancer treatments have long been moving toward personalization—finding the right drugs that work for a patient’s unique tumor, based on specific genetic and molecular patterns. Many of these targeted therapies are highly effective, but aren’t available for all cancers, including non-small cell lung cancers (NSCLCs) that have an LKB1 genetic mutation.
A new study led by Salk Institute Professor Reuben Shaw and former postdoctoral fellow Lillian Eichner, now an assistant professor at Northwestern University, revealed FDA-approved trametinib and entinostat (which is currently in clinical trials) can be given in tandem to produce fewer and smaller tumors in mice with LKB1-mutated NSCLC.
The findings were published in Science Advances on March 17, 2023.
“For non-small cell lung cancer cases with the LKB1 mutation, standard chemotherapy and immunotherapy treatments are not effective,” says Shaw, senior and co-corresponding author of the study, and director of Salk’s Cancer Center. “Our findings demonstrate there is a way to target these cases using drugs that are FDA-approved or already in clinical trials, so this work could easily be used for a clinical trial in humans.”
Roughly 20 percent of all NSCLCs have the LKB1 genetic mutation, which means there are no effective targeted therapies currently on the market for patients with this cancer type. To create a therapy that could target the LKB1 mutation, the researchers turned to histone deacetylases (HDACs). HDACs are proteins associated with tumor growth and cancer metastasis, with characteristic overexpression in solid tumors.
Several HDAC-inhibitor drugs are already FDA-approved (safe for human use) for specific forms of lymphoma, but data on their efficacy in solid tumors or whether tumors bearing specific genetic alterations may exhibit heightened therapeutic potential has been lacking.
Based on previous findings connecting the LKB1 gene to three other HDACs that all rely on HDAC3, the team started by conducting a genetic analysis of HDAC3 in mouse models of NSCLC, discovering an unexpectedly critical role for HDAC3 in multiple models. After establishing that HDAC3 was critical for the growth of the difficult-to-treat LKB1-mutant tumors, the researchers next examined whether pharmacologically blocking HDAC3 could give a similarly potent effect.
The team was curious about testing two drugs, entinostat (an HDAC inhibitor in clinical trials known to target HDAC1 and HDAC3) and FDA-approved trametinib (an inhibitor for a different class of enzymes related to cancer). Tumors often become quickly resistant to trametinib, but co-treatment with a drug that inhibits a protein downstream of HDAC3 helps reduce this resistance.
Because that protein relies on HDAC3, the researchers believed that a drug that targets HDAC3—like entinostat—would help manage trametinib resistance, too.
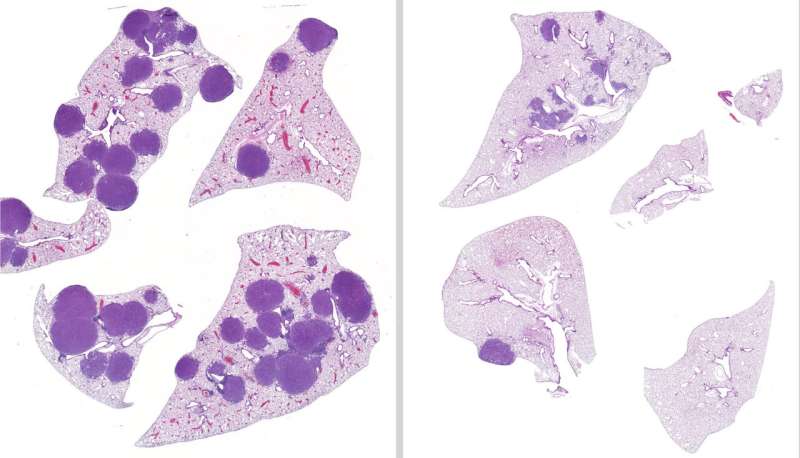
After treating mice with LKB1-mutated lung cancer with variable treatment regimens for 42 days, the team found that mice given both entinostat and trametinib had 79 percent less tumor volume and 63 percent fewer tumors in their lungs than the untreated mice. Additionally, the team confirmed that entinostat was a viable treatment option in cases where a tumor was resistant to trametinib.
“We thought the whole HDAC enzyme class was directly linked to the cause of LKB1 mutant lung cancer. But we didn’t know the specific role of HDAC3 in lung tumor growth,” says first and co-corresponding author Eichner. “We’ve now shown that HDAC3 is essential in lung cancer, and that it is a druggable vulnerability in therapeutic resistance.”
The findings may lead to clinical trials to test the new regimen in humans, since entinostat is already in clinical trials and trametinib is FDA-approved. Importantly, Shaw sees this discovery as transformative for cancers beyond NSCLC, with potential applications in lymphoma, melanoma, and pancreatic cancer.
“Our lab has committed years to this project, taking small and meaningful steps toward these findings,” says Shaw, holder of the William R. Brody Chair. “This is truly a success story for how basic discovery science can lead to therapeutic solutions in the not-so-distant future.”
“My independent laboratory is fortunate to be part of the Lurie Cancer Center at the Feinberg School of Medicine at Northwestern University, which is very supportive of translational research. We hope that this environment will facilitate the initiation of a clinical trial based on these findings,” says Eichner.
More information:
Lillian Eichner et al, HDAC3 is critical in tumor development and therapeutic resistance in Kras-mutant non–small cell lung cancer, Science Advances (2023). DOI: 10.1126/sciadv.add3243. www.science.org/doi/10.1126/sciadv.add3243
Journal information:
Science Advances
Source: Read Full Article


