The most recent statistics put the number of regular cocaine or crack cocaine users in the world at 20 million. Of these, one in four will become addicted or develop use disorders.
Among the addicts, many are women who, when they become pregnant, can bring risks to themselves and their children. Cocaine use during pregnancy is associated with serious conditions for pregnant women (such as severe pre-eclampsia or miscarriage) and babies (premature birth with complications, low birth weight, malformations and withdrawal syndrome in the newborn).
A study carried out by a research team I am part of at Universidade Federal de Minas Gerais (UFMG), in Brazil, has achieved a breakthrough: a vaccine that uses the immune system to prevent the perinatal consequences of drug use, and which could protect the children of drug-using mothers. If clinical studies prove the vaccine’s efficacy, it could be an important tool to complement the biopsychosocial treatments already used to treat people with cocaine and crack addictions. Our research is published in the Journal of Medicinal Chemistry.
Pre-clinical tests with the new vaccine on animals have already been carried out successfully. At the moment, the research project is trying to get this experimental drug registered with the Brazilian Health Regulatory Agency Anvisa so that clinical trials can begin and is looking for funds to carry them out.
More than a decade in the making
During my doctorate, I studied how our bodies produce antibodies that contribute to the perception of certain symptoms of depression. These antibodies, induced by intestinal bacteria, are capable of changing the action of certain hormones and neurotransmitters and changing our perception, creating symptoms and modifying our perception. In 2011, when I applied to UFMG, I started working on the idea of using this knowledge to produce a vaccine against cocaine.
A few years earlier, an American group had published articles showing that cocaine produced antibodies in some addicts who used very large quantities of the drug. They then began studies with the aim of using this mechanism, which we can call a self-defensive mechanism of the organism, to help people with addictions to cocaine and its derivatives, such as crack.
I was already working on the vaccine when scientific research ran into reality: a tragic situation we experienced in Minas Gerais state became another trigger for the vaccine’s development. In 2013, the Public Prosecutor’s Office issued a rule to the Family Courts, obliging doctors to notify cases of births to drug-dependent women, which would take the newborn children into the adoption system. Immediately, hundreds of women arrived at the drug addiction clinic at UFMG’s Hospital das Clínicas, asking for help so they wouldn’t lose custody of their children.
It was a very sad situation. Motherhood is a time of conflict for these women, who want to protect the baby, but often can’t avoid the compulsion to use the drug. Only 25 percent of them manage to stop using during pregnancy.
But science is also made up of encounters. When I raised the issue with Professor Ângelo de Fátima, one of Brazil’s leading experts in medicinal chemistry, he offered to replicate the molecule that the Americans had produced so that we could try an experiment on pregnant rats.
A while later we talked about a new type of molecule, which had produced an immunogenic response to cancer, and from there came an innovation, a totally synthetic molecule, which we now call UFMG-V4N2.
After discussing the possible immunogenicity—the ability to induce an immune response—of molecular structures called calixarenes, we agreed that he would synthesize a new molecule, called UFMG-V4N2, which is the basis of the Calixcoca vaccine. UFMG-V4N2 is actually a vaccine platform that can virtually be used to produce other vaccines, against methamphetamine, opioid and nicotine addiction, for example, for which we already have molecule designs under study.
Protected pregnancy
In the study with the rats, we observed that the vaccination induced the production of antibodies in the pregnant animals, which is a major challenge: pregnancy is a state in which the body’s immune response is lower, so that the baby is not considered a foreign body. The production of antibodies in pregnant women is therefore usually more complicated and, in the case of cocaine users, the drug itself already has an immunosuppressive effect.
Given the presence of the two immunosuppressive effects, we thought it would be very difficult for the mechanism to work. But paradoxically, the antibody production response was almost a thousand times greater than in the male mice. It’s a paradox for which we don’t yet have an immunological explanation, but it’s a finding that opens up a very interesting window of opportunity if this mechanism is reproduced in humans.
The vaccinated rats did not experience the lack of appetite or hyperactivity induced by cocaine, and had 30 percent more offspring than the unvaccinated rats, which indicates a reduction in miscarriages, placental abruption and other perinatal complications. The antibodies produced were able to block the passage of the drug through the placenta, protecting the mice. Furthermore, we identified that the antibodies also pass through the milk, so when breastfeeding, the women, even when using the drug, may not cause harm to the babies.
Addiction
In addition to the benefits of the vaccine for pregnant women and their children, we believe that Calixcoca could also become an important tool to add to the addiction treatment package, which should also involve psychiatric, psychological and social care support and help from the family.
There are medicines that help with other addictions, such as alcohol or tobacco, but not so far for crack and cocaine, which are the drugs that most stimulate the brain’s reward circuit and, consequently, have a very high addictive power. Only 20 percent of patients who undergo treatments manage to be drug-free within five years, which is a pretty poor result.
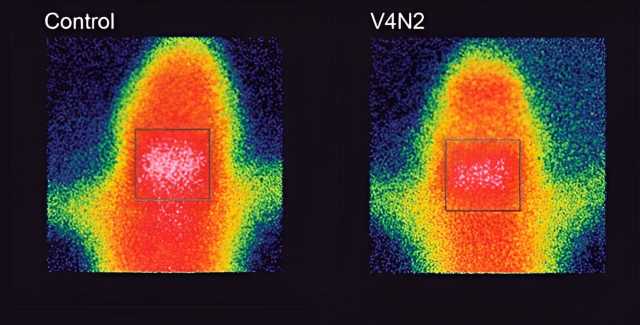
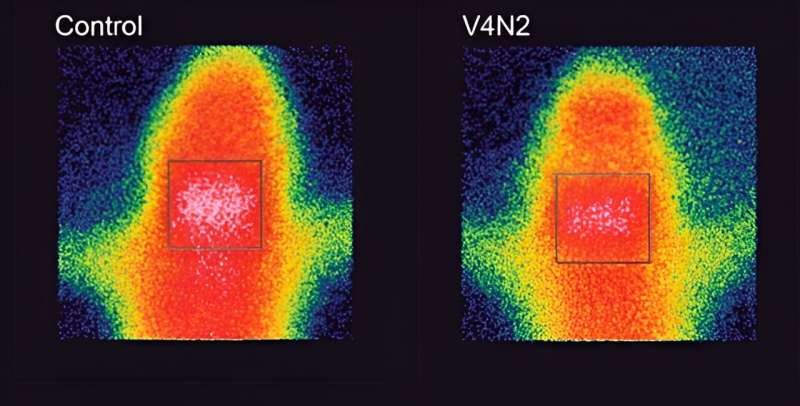
UFMG-V4N2 proved effective in producing antibodies and making them block the passage of cocaine into the brain, which means that the vaccinated animals have a reduced perception of the drug’s effect: a very important advantage in a treatment.
This blockage occurs in the following way: we have a “protective shield” called the blood-brain barrier, which prevents toxic elements, viruses or bacteria from entering the brain, but because the cocaine molecule is very small, it manages to pass through this barrier.
The vaccine stimulates the production of antibodies, which bind to the drug molecules, increasing their weight and size and thus preventing them from getting past the protective shield. The cocaine is retained in the blood, but as it is bound to the antibody, it doesn’t act on the heart or arteries either, which means the risk of overdoses is reduced.
Cocaine and crack cocaine addiction is an extremely important medical, psychological and social problem that still has no definitive solution. Even before human trials begin, around 3,500 people have already contacted us spontaneously, interested in taking part as volunteers in clinical studies.
That’s why the results achieved so far are so relevant: there is no treatment approved by regulatory agencies worldwide for this purpose, and Calixcoca could represent hope for thousands of users who want to quit the drug but can’t avoid relapsing. We still have a long way to go to complete the development of this treatment, which could contribute to improving the psychosocial treatments currently used to care for people suffering from cocaine and crack addiction.
More information:
Rachel J. Stephenson et al, Anti-cocaine Vaccine Development: Where Are We Now and Where Are We Going?, Journal of Medicinal Chemistry (2023). DOI: 10.1021/acs.jmedchem.3c00366
Journal information:
Journal of Medicinal Chemistry

Source: Read Full Article