A retrospective surveillance study of Pfizer–BioNTech BNT162b2 mRNA COVID-19 vaccination in Israel
The coronavirus disease 2019 (COVID-19), which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has directly affected over 232 million cases and caused over 4.76 million deaths worldwide.
One of the first countries that implemented a nationwide vaccination campaign against COVID-19 was Israel, which was led by the Israel Ministry of Health. The campaign started on December 2020 and intended to administer two doses of the Pfizer–BioNTech BNT162b2 messenger ribonucleic acid (mRNA) COVID-19 vaccine 21 days apart to all citizens who were 16 years old or above.
 Study: Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer–BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: a retrospective surveillance study. Image Credit: guteksk7 / Shutterstock.com
Study: Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer–BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: a retrospective surveillance study. Image Credit: guteksk7 / Shutterstock.com
Vaccine campaign in Israel
The population of Israel is approximately 9.2 million, out of which 6.5 million are 16 years and above. The vaccine was first administered to target groups that comprised healthcare workers, immunocompromised individuals, long-term care facility residents, and individuals who were aged 60 years and above.
It was reported that by April 2021, 10 million doses of the vaccine were administered in Israel. More than 70% of the Israelis aged 16 years and older had received two doses of the vaccine, with more than 90% of adults aged 65 years and older receiving two doses of the vaccine.
The vaccination program was found to coincide with a large wave of SARS-CoV-2. This resulted in the implementation of a nationwide lockdown on December 27, 2020, a week after the vaccination program had started. The daily infections peaked during this time, with more than 10,000 cases reported on January 20, 2020.
The lockdown restrictions started to ease from February 7, 2020 and were eventually lifted on March 7, 2020. Following the lifting of lockdown restrictions, COVID-19 cases remained low until June 23. 2020. On June 24, 2020, the number of cases was found to increase again due to the introduction of the Delta variant of concern into the country.
The high vaccine uptake in Israel during the time of high SARS-CoV-2 circulation has helped to determine the real-world effectiveness of the BNT162b2 vaccine. However, the number of SARS-CoV-2 infections, as well as the COVID-19-related hospitalizations and deaths that could be avoided due to vaccination, have yet to be determined. One recent study provided nationwide estimates of the burden of SARS-CoV-2 that could be averted by Israel's vaccination campaign.
About the study
The current study published in The Lancet journal took place between December 20, 2020, and April 10, 2021, and involved a randomized controlled trial of BNT162b2. Individuals aged 16 years or older who had received at least one dose of the vaccine were recruited in the study. A follow-up period of at least 14 days was also involved; therefore, the analysis of the study began on January 3, 2020, 14 days after the start of the vaccination program.
Furthermore, countrywide daily aggregate data of reverse-transcriptase polymerase-chain-reaction (RT-PCR)-confirmed SARS-CoV-2 positive cases were obtained from the national database of the Ministry of Health, along with data on vaccine uptake. RT-PCR testing for SARS-CoV-2 was free of cost and widely available in Israel. Individuals who had close contact with COVID-19 infected individuals, who were returning from travel abroad, and who exhibited certain symptoms of COVID-19 were advised to undergo the RT-PCR test for confirmation.
The study estimated the averted burden of four outcomes: SARS-CoV-2 infections and COVID-19-related hospitalizations, severe hospitalization, critical hospitalization, and death. Hospitalizations were considered to be severe if the patient had an oxygen saturation on room air of less than 94%, resting respiratory rate of more than 30 breaths per min, or ratio of arterial partial pressure of oxygen to fraction of inspired oxygen of less than 300 mmHg. Hospitalizations were considered critical if they involved mechanical ventilation, cardiac, renal, or hepatic failure, or shock.
Study findings
The results of the study indicate that by April 10, 2021, 80% of Israel's population aged 16 years or above were partially vaccinated and 74% were fully vaccinated. The vaccine coverage was found to be greater in individuals aged 65 years or above.
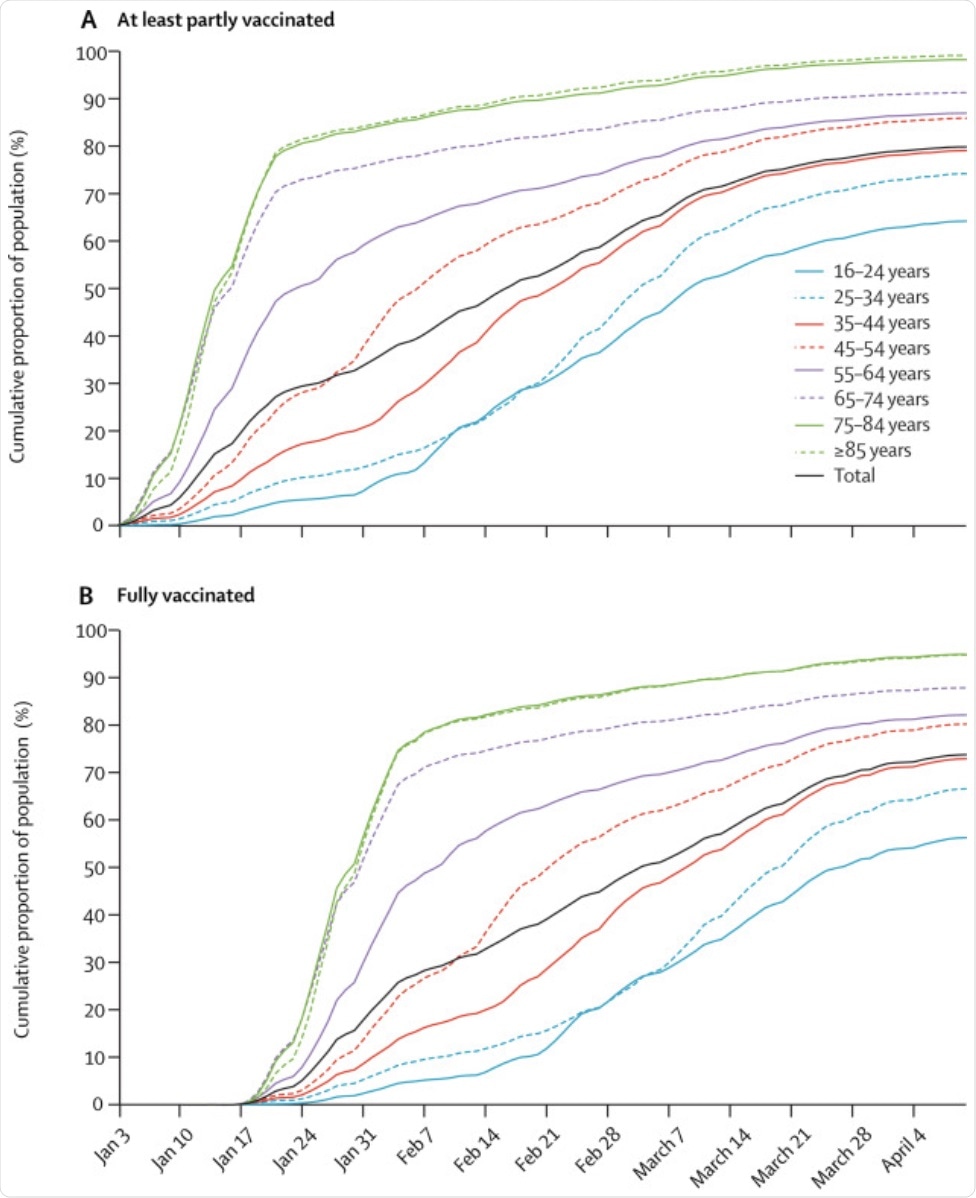 Cumulative proportion of the population who were at least partly vaccinated (A) and who were full vaccinated (B). Dec 20, 2020, was the start of Israel's nationwide vaccination campaign, but the analysis period started on Jan 3, 2021, which was the first day on which people could be at least partly vaccinated (defined as individuals who received at least one dose of BNT162b2 with at least 14 days of follow-up after the first dose). Fully vaccinated individuals are people who received two doses of vaccine with at least 7 days of follow-up after the second dose. Week time points are indicted with the week starting on a Sunday.
Cumulative proportion of the population who were at least partly vaccinated (A) and who were full vaccinated (B). Dec 20, 2020, was the start of Israel's nationwide vaccination campaign, but the analysis period started on Jan 3, 2021, which was the first day on which people could be at least partly vaccinated (defined as individuals who received at least one dose of BNT162b2 with at least 14 days of follow-up after the first dose). Fully vaccinated individuals are people who received two doses of vaccine with at least 7 days of follow-up after the second dose. Week time points are indicted with the week starting on a Sunday.
Since the onset of the pandemic, Israel had reported a total of 835,811 SARS-CoV-2 infections and 6,292 COVID-19-related deaths until April 10, 2021. Of these, 269,459 SARS-CoV-2 infections, 13,338 COVID-19-related hospitalizations, 8,429 severe or critical COVID-19-related hospitalizations, and 2,859 COVID-19-related deaths took place between January and April 2021 in individuals 16 years old or above. It was also determined that vaccination averted 158,665 SARS-CoV-2 infections, 24,597 COVID-19-related, hospitalizations, 17,432 severe or critical COVID-19-related hospitalizations, and 5,532 COVID-19-related deaths.
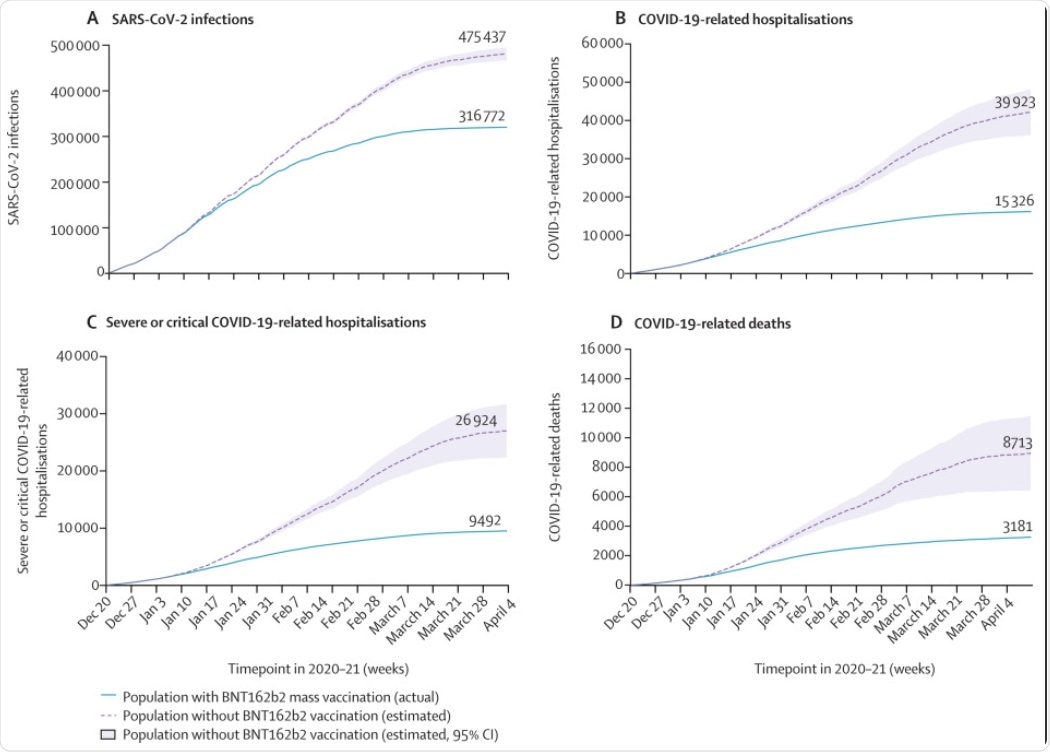 Cumulative SARS-CoV-2 outcomes over time comparing observed cases with nationwide vaccination and predicted cases without vaccination by outcome, Dec 20, 2020, to April 10, 2021. (A) SARS-CoV-2 infections. (B) COVID-19-related hospitalisations. (C) Severe and critical COVID-19-related hospitalisations. (D) COVID-19-related deaths. Lines are estimates over time with shaded areas showing 95% CIs.
Cumulative SARS-CoV-2 outcomes over time comparing observed cases with nationwide vaccination and predicted cases without vaccination by outcome, Dec 20, 2020, to April 10, 2021. (A) SARS-CoV-2 infections. (B) COVID-19-related hospitalisations. (C) Severe and critical COVID-19-related hospitalisations. (D) COVID-19-related deaths. Lines are estimates over time with shaded areas showing 95% CIs.
Thus, the results indicate that Israel would have at least three times more hospitalizations and deaths if the vaccination campaign did not take place. Furthermore, the study also found that 73% of SARS-CoV-2 infections and 79% COVID-19-related hospitalizations and deaths could be averted in fully vaccinated individuals.
“These data highlight the direct effect of the recommended two-dose schedule, but also elucidate the additional, incremental benefit of protection after only one dose while awaiting completion of the full two-dose schedule.”
Limitations
Although the study was effective in determining the importance of the vaccination campaign, it had certain limitations. The analysis was stratified based on only age and date but did not include other important factors such as comorbidities, the likelihood of getting tested, or socioeconomic status between vaccination and unvaccinated individuals.
The analysis also did not include the potential indirect effects of the vaccine on the unvaccinated population. The analysis was conducted when the SARS-CoV-2 Alpha variant was in circulation in Israel; therefore, further modeling needs to be done when other variants of concerns arise.
Individuals with previous SARS-CoV-2 infections were excluded from the analysis. Also, since the timing of the vaccination campaign and nationwide lockdown coincided, it was difficult to differentiate the effects of vaccination from that of lockdown restrictions.
- Haas, E. J., McLaughlin, J. M., Khan, F.,. et al. (2021). Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer–BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: a retrospective surveillance study. The Lancet Infectious Diseases. doi:10.1016/S1473-3099(21)00566-1. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00566-1/fulltext
Posted in: Medical Research News | Disease/Infection News | Healthcare News | Pharmaceutical News
Tags: Coronavirus, Coronavirus Disease COVID-19, Efficacy, Healthcare, Oxygen, Pandemic, Polymerase, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine

Written by
Suchandrima Bhowmik
Suchandrima has a Bachelor of Science (B.Sc.) degree in Microbiology and a Master of Science (M.Sc.) degree in Microbiology from the University of Calcutta, India. The study of health and diseases was always very important to her. In addition to Microbiology, she also gained extensive knowledge in Biochemistry, Immunology, Medical Microbiology, Metabolism, and Biotechnology as part of her master's degree.
Source: Read Full Article


