
Researchers at Kumamoto University, Japan, have generated mice lacking the estrogen receptor beta (ERβ) gene, both fiber-specific and muscle stem cell-specific, which resulted in abnormalities in the growth and regeneration of skeletal muscle in female mice. This was not observed in male mice that lacked the ERβ gene, suggesting that estrogen and its downstream signals may be a female-specific mechanism for muscle growth and regeneration.
In humans, skeletal muscle mass generally peaks in the 20s with a gradual decline beginning in the 30s, but it is possible to maintain muscle mass through strength training and a healthy lifestyle. Skeletal muscle can be damaged through excessive exercise or bruising, but it has the ability to regenerate. The muscle stem cells that surround muscle fibers are essential for this regeneration; they also play a part in increasing muscle size (hypertrophy). Muscle stem cell dysfunction is thought to be associated with various muscle weakness, such as age-related sarcopenia and muscular dystrophy. Although basic research on skeletal muscle has progressed rapidly in recent years, most studies were conducted on male animals and gender differences were given much consideration.
Estrogen is a female hormone that maintains the homeostasis of various tissues and organs. A decrease in estrogen levels due to amenorrhea, menopause, or other factors can lead to a disturbance in biological homeostasis. When estrogen binds to estrogen receptors (ERs) in cells, it is transferred into the nucleus and binds to genomic DNA to induce the expression of specific genes as transcription factors. There are two types of ERs, ERα and ERβ. While both ERα and ERβ have high binding capacity to estrogen, their tissue distribution is different, they do not have a common DNA-binding domain, and they may act as antagonists to each other, suggesting that they have different roles. Furthermore, estrogen’s effects on cells can be both ER-mediated and non-ER-mediated.
An epidemiological study of pre- and postmenopausal women in their 50s indicated an association between decreased blood estrogen levels and muscle weakness. A research group at Kumamoto University previously showed that estrogen is important for skeletal muscle development and regeneration using an ovariectomized estrogen deficiency mouse model. They also examined the effectiveness of nutritional interventions in estrogen-deficient conditions. However, whether estrogen acts directly on the ER of muscle fibers and muscle stem cells to regulate skeletal muscle growth and regeneration, or whether it acts indirectly through other tissues and organs was unclear. In this study, the researchers generated mice with either myofiber-specific or muscle stem cell-specific ERβ gene deletion and analyzed the function of ERβ in skeletal muscle.
To clarify the role of ERβ in the growth of skeletal muscle, researchers generated mice (mKO) in which the action of the ERβ gene could be turned off in myofibers with the administration of the drug doxycycline. ERβ deficiency was induced at six weeks of age, and muscle fiber area and strength of the tibialis anterior muscle was measured at 10 to 12 weeks. Compared to control mice, both indices were reduced in female mKO mice but not in male mice. Since there was no change in the expression of muscle atrophy-related genes, this reduced growth of female mice was not thought to be due to an increase in muscle atrophy. Ovariectomy-induced estrogen deficiency is known to be associated with muscle quality changes, such as a relative increase in the proportion of fast-type fibers, but no such qualitative changes were observed in mKO mice. It was therefore suggested that, while it may have a direct effect on myofiber growth via ERβ (as expressed in myofibers), estrogen may also regulate the quality of myofibers in a non-ERβ-mediated manner.
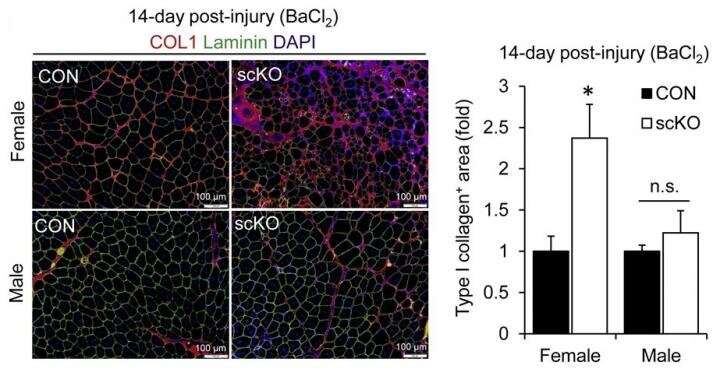
To determine the function of ERβ in muscle stem cells, the researchers generated scKO mice in which the ERβ gene could be deleted in muscle stem cells with the administration of the drug tamoxifen. They then evaluated muscle regenerative capacity by locally inducing muscle damage. While muscle regeneration was efficient in control mice, the regenerated muscle tissue of female scKO mice showed thin regenerated muscle fibers, fibrosis caused by collagen deposition, and significantly reduced muscle regenerative capacity. Muscle regeneration in male scKO mice, however, was not impaired. Because impaired muscle regeneration in females was not exacerbated by ovariectomies that made them estrogen deficient, the researchers thus thought that estrogen regulates muscle regeneration via ERβ expressed by muscle stem cells.
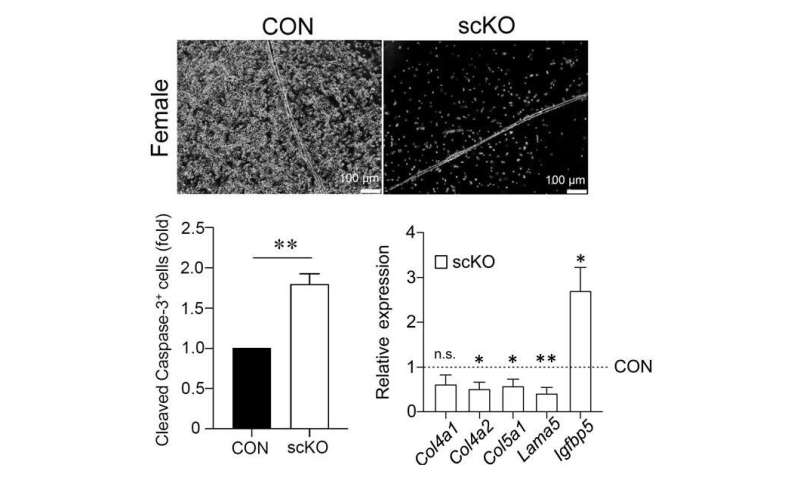
To further investigate the cause of reduced muscle regenerative capacity, researchers isolated and cultured muscle stem cells for evaluation. ERβ in cells from scKO mice was evaluated in several experiments using siRNAs and inhibitors. ERβ was found to contribute to the promotion of muscle stem cell proliferation and the inhibition of cell death. Gene expression analysis (RNA-seq) of scKO muscle stem cells showed that the expression of “niche”-related genes, which are required for the maintenance of stem cell properties, was reduced in scKO muscle stem cells. Therefore, the researchers hypothesize that the inactivation of ERβ may have affected the proliferation and survival of muscle stem cells by inhibiting the formation of stem cell niches.
This study is thought to be the first to show that ERβ in genetic mouse models plays an important role in the growth and regeneration of skeletal muscle through its function in both muscle fibers and muscle stem cells. However, the role of ERβ in male mice has not yet been elucidated and remains to be addressed even though its expression in both male and female mice is comparable.
Source: Read Full Article
