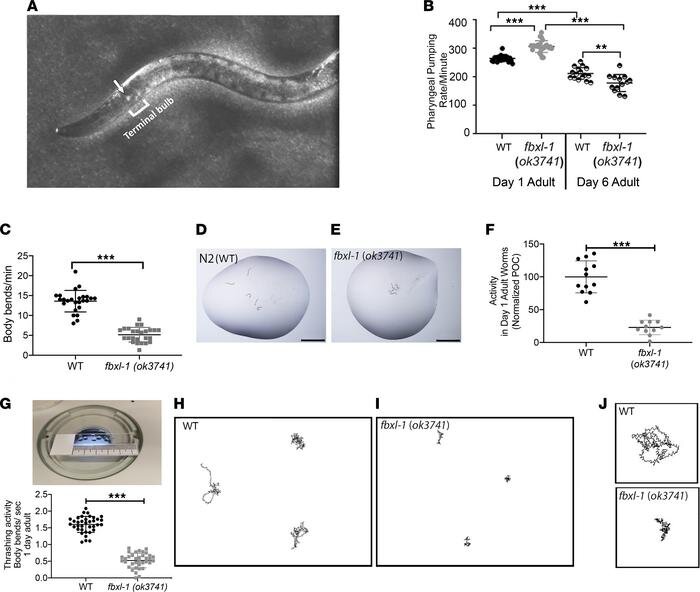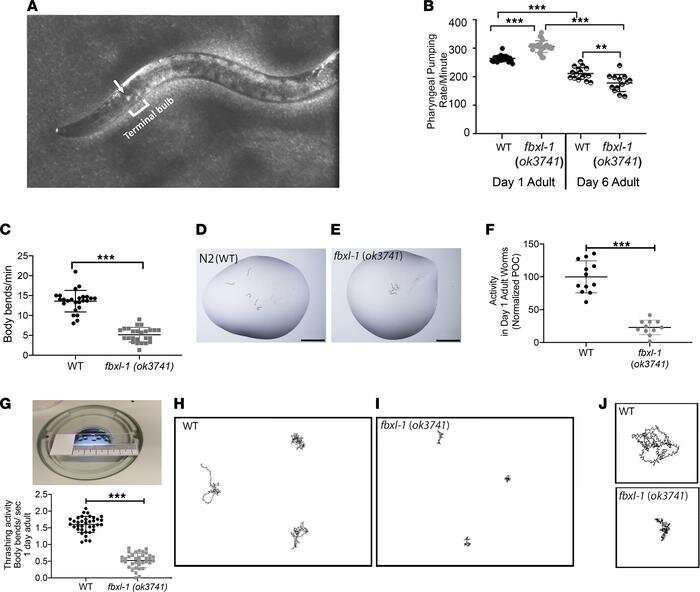
Researchers at Children’s Hospital of Philadelphia (CHOP) have discovered a molecule that may have therapeutic benefits for children with a life-threatening form of mitochondrial disease. The findings were published in the journal JCI Insight.
Mitochondrial disease can describe a wide variety of conditions characterized by different symptoms and a range of severity. However, all of these conditions are linked by issues related to the mitochondria. Primary mitochondrial diseases are a growing class of genetic-based mitochondrial disorders, caused by inherited mutations in any of several hundred different genes.
One particularly severe form of primary mitochondrial disease is caused by variant in the F-Box and Leucine Rich Repeat Protein 4 (FBXL4) gene. At a microscopic level, this disorder disrupts the mitochondrial respiratory chain complex that converts oxygen and nutrients into energy, while also causing a buildup of lactic acid. The condition affects multiple systems in the body and can result in several symptoms including weakened muscle tone, slowed growth, developmental delays, impairment of motor skills and speech, seizures, and renal problems. Infants diagnosed with this disorder often only live into early to mid-childhood, prompting the need to investigate novel therapeutic interventions for these critically ill patients.
“These are typically very sick children. Since the gene that caused this form of mitochondrial disease was discovered by our research group a decade ago, we have been striving to find an intervention of true therapeutic value,” said senior study author Marni Falk, MD, Professor of Pediatrics and Executive Director of the Mitochondrial Medicine Program at CHOP. “This study is truly the culmination of translating basic research into clinically relevant findings that represents an enormous breakthrough in understanding how we might be able to improve the health of these patients, along with the breadth of cellular effects that treatment may have.”
In this study, researchers looked at 12 potential drug candidates and tested their effectiveness in newly created microscopic roundworm C. elegans models with FBXL4 gene pathogenic variants. Of these candidates, dichloroacetate (DCA) demonstrated clear beneficial effects on neuromotor activity and mitochondrial function. Validation studies were then performed both in zebrafish animal models and in human FBXL4 patient fibroblast cell lines, where DCA prevented stressor-induced brain death, impairment of neurologic and muscular functions, mitochondrial dysfunction, and other stressor-induced mitochondrial defects.
Source: Read Full Article