Vaccine-induced antibodies still potent against more contagious SARS-CoV-2 variant
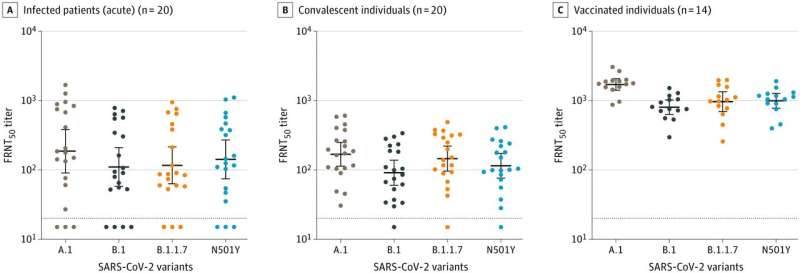
The variant of the SARS-CoV-2 virus known as B.1.1.7 is still susceptible to antibodies induced after COVID-19 infection or immunization with a currently available vaccine, according to Emory University scientists.
The increasingly prevalent B.1.1.7 variant was first identified in the United Kingdom, and was detected in the United States in December 2020. It is thought to be more contagious than the strain of SARS-CoV-2 that initially emerged at the beginning of 2020.
The Emory researchers’ results are reassuring—at least with respect to the B.1.1.7 variant. They mean that people who had COVID-19 early in the pandemic, or have been vaccinated already, will likely not face a handicap against the B.1.1.7 form of the virus.
Experiments to test other emerging SARS-CoV-2 variants against infection- and vaccine-induced antibodies are ongoing in the laboratory of virologist Mehul Suthar, Ph.D., who is lead author of the current report in the Journal of the American Medical Association.
“The B.1.1.7 variant appears to replicate at higher levels in the respiratory tract,” Suthar says. “One of the mutations—the D614G spike mutation—took over sometime during the summer in many places, because viruses carrying it out-competed others. However, the B.1.1.7 mutations appear not to affect the potency of the neutralizing antibodies we obtained.”
Neutralizing antibodies refers to antibodies present in the blood that can prevent SARS-CoV-2 from being able to infect cells in culture. To test them, researchers in Suthar’s laboratory use a live virus assay, which is thought to more accurately reflect the cellular context of antibodies as they interact with infectious virus.
Live virus assays are conducted under Biosafety Level 3 (BSL3) conditions, in contrast with pseudovirus assays, which mix SARS-CoV-2 genes into other viruses, and can be performed more conveniently in the laboratory.
Suthar is assistant professor of pediatrics at Emory University School of Medicine and Emory Vaccine Center, and his laboratory is based at Yerkes National Primate Research Center. The first author of the paper is postdoctoral fellow Venkata Viswanadh Edara, Ph.D.
The antibody samples came from 14 adults who participated in the phase 1 Moderna mRNA-1273 vaccine study in 2020, along with 20 acutely infected COVID-19 patients and 20 people who were a few months past their COVID-19 illnesses. Samples from the Moderna vaccine trial were provided by the National Institute of Allergy and Infectious Diseases.
Suthar says that the bank of antibody samples from patients and vaccinated individuals will be valuable as his laboratory continues to test other viral variants.
“We’re going to continue to test more variants as they emerge,” he says. “We need to monitor them as they evolve.”
In the laboratory, both infection- and vaccine-induced antibodies were effective in neutralizing the SARS-CoV-2 B.1.1.7 variant virus—on par with a virus sample obtained from a patient from the Seattle area early in 2020, which was used as a reference.
Source: Read Full Article



