Pfizer Requests FDA Approval to Use Its COVID Vaccine in Kids Ages 12 to 15
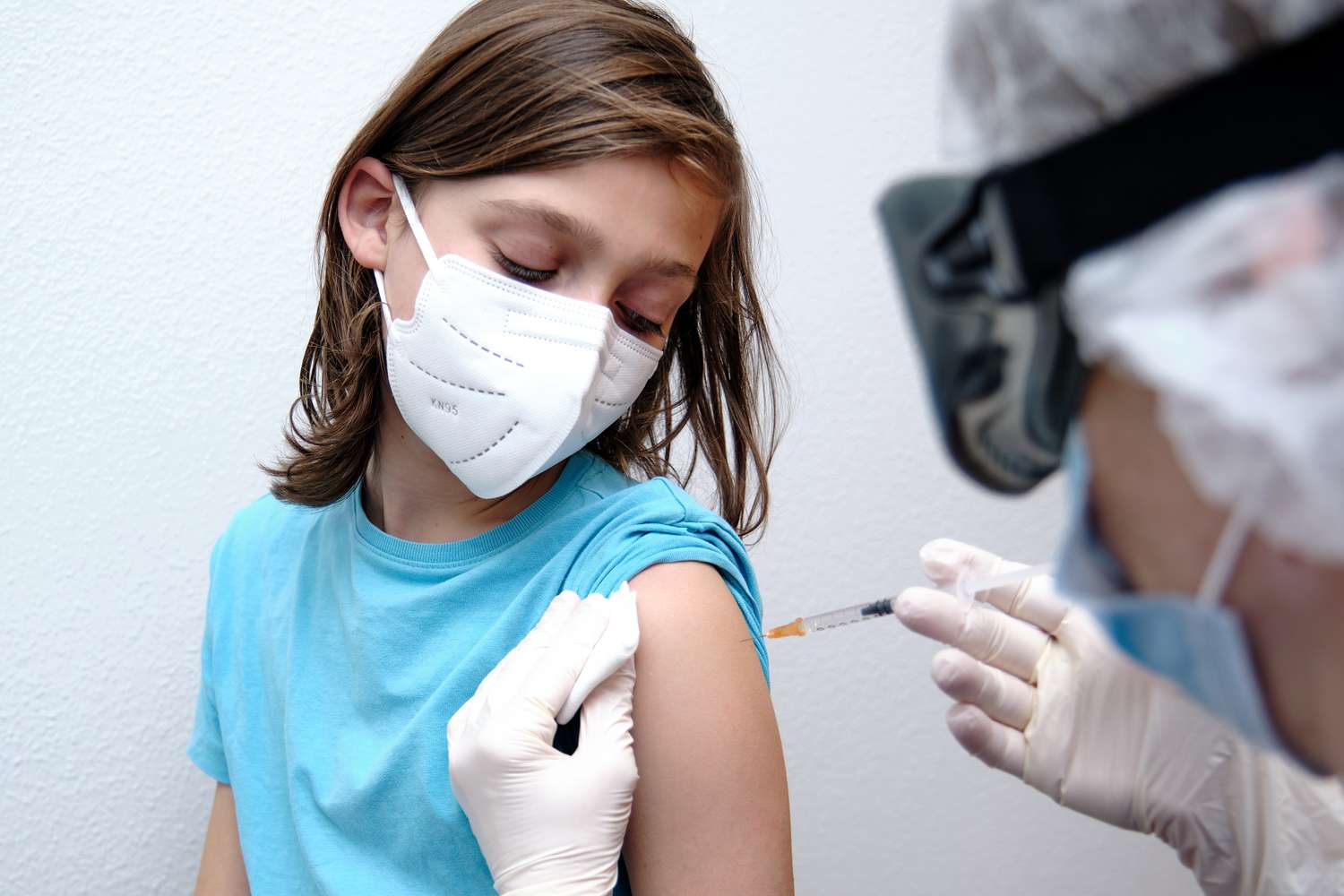
After trial results showed that its vaccine is 100% effective in preventing COVID-19 in kids, Pfizer-BioNTech has formally asked the Food and Drug Administration for approval to begin vaccinating children ages 12 to 15.
The company requested Friday that the FDA amend its emergency use authorization to lower the approved age for vaccination from 16 years old to 12. In a statement, Pfizer said that it will make the same request to other countries currently using the vaccine in the coming days, CNN reported.
"These requests are based on data from the pivotal Phase 3 trial in adolescents 12 to 15 years of age with or without prior evidence of SARS-CoV-2 infection [the virus that causes COVID-19 illness], which demonstrated 100% efficacy and robust antibody response after vaccination with the COVID-19 vaccine," the company said.
Pfizer had announced the strong results from clinical trials in adolescents on March 31, adding that the vaccine was "well tolerated" by the 12-to-15-year-olds, with "robust antibody responses" even "exceeding those recorded earlier in vaccinated participants ages 16 to 25 years old."
Pfizer Chairman and CEO Albert Bourla said in a statement that the company hopes to start "to vaccinate this age group before the start of the next school year."
Along with testing their vaccines in adolescents, both Pfizer and Modern are currently running clinical trials on their vaccines in children between the ages of 6 months and 12 years old.
Currently, Pfizer's vaccine is approved for use in people ages 16 and up, while the other two vaccines currently in use in the U.S., from Moderna and Johnson & Johnson, are approved for people 18 and older.
As of April 9, one in four adults in the U.S. have been fully vaccinated, while more than a third of the population, 112,046,611 people, have received at least one dose of a vaccine, according to the Centers for Disease Control. Nearly every state has also opened vaccine eligibility to anyone ages 16 and up, ahead of President Joe Biden's stated goal of April 19.
As information about the coronavirus pandemic rapidly changes, PEOPLE is committed to providing the most recent data in our coverage. Some of the information in this story may have changed after publication. For the latest on COVID-19, readers are encouraged to use online resources from CDC, WHO, and local public health departments. PEOPLE has partnered with GoFundMe to raise money for the COVID-19 Relief Fund, a GoFundMe.org fundraiser to support everything from frontline responders to families in need, as well as organizations helping communities. For more information or to donate, click here.
Source: Read Full Article


