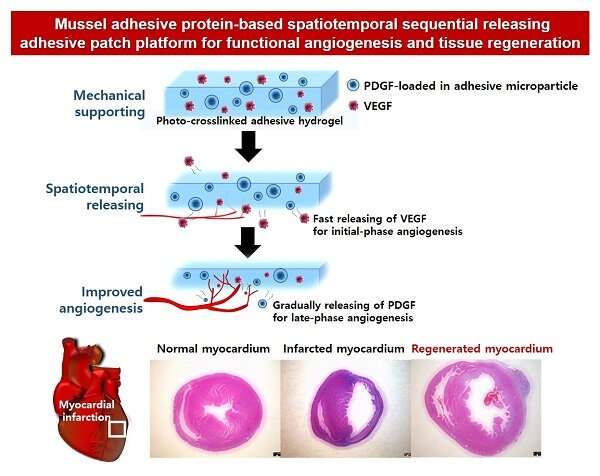
Blood vessels deliver nutrients and oxygen to each organ in our body. They are difficult to completely restore to their original conditions once damaged by myocardial infarction or severe ischemic diseases. This is because various angiogenic growth factors must be applied sequentially in order to restore the vascular structure. Recently, a research team at POSTECH has bioengineered a novel adhesive patch platform that can efficiently deliver blood vessel-forming growth factors spatiotemporally using mussel adhesive protein (MAP), a bio-adhesive material that is made from mussels harmless to humans.
A POSTECH research team led by Professor Hyung Joon Cha and Dr. Tae Yoon Park of the Department of Chemical Engineering has developed a drug-delivering adhesive patch using two formulations—coacervate-based microparticles and light activation-based crosslinked hydrogels—that can be mass-produced. The team verified their effectiveness in tissue regeneration including neovascularization using a rat model for myocardial infarction and excisional wound model incapable of self-healing.
The research team produced microparticles by instantaneously encapsulating and crosslinking platelet-derived growth factor (PDGF) required for the late-phase angiogenesis during the process of forming coacervate microdroplets formed by the electrostatic interaction of MAP and hyaluronic acid.
In addition, PDGF-loaded microparticles and the vascular endothelial growth factor (VEGF) required for initial-phase angiogenic factor simultaneously encapsulated in a MAP-based crosslinked hydrogel, spatially separated and mounted the two factors easily and quickly.
This effective novel angiogenesis-inducing platform showed that PDGF was secreted and delivered 1.9 times later in time despite its smaller size than VEGF. In addition, the team confirmed that the platform allows crosslinking to occur instantaneously so that it can easily adhere even to uneven curved surfaces.
“We have developed a new platform that can efficiently deliver angiogenic factors spatiotemporally by using the formulation property of MAP, an original biomaterial,” remarked Professor Hyung Joon Cha who has long devoted himself to studying the MAP. “It is of great significance that we have confirmed the functional recovery beyond effective neovascularization by applying it to an actual animal model of myocardial infarction and severely damaged skin model.”
Source: Read Full Article
