A recent phase 1/2 study under review at the Nature Portfolio journal and posted to the Research Square* preprint server demonstrated that a peptide-centered severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) T-cell activator, CoVac-1, was efficient in B-cell deficient people.
 Study: Phase I/II trial of a peptide-based COVID-19 T-cell activator in patients with B-cell deficiency. Image Credit: Kateryna Kon / Shutterstock
Study: Phase I/II trial of a peptide-based COVID-19 T-cell activator in patients with B-cell deficiency. Image Credit: Kateryna Kon / Shutterstock
Background
The SARS-CoV-2-induced coronavirus disease 2019 (COVID-19) pandemic prompted the invention of numerous vaccinations that safeguard billions of humans from the disease's severe course, primarily through the generation of humoral or antibody-triggered immunity. T-cell immunity is critical for controlling SARS-CoV-2 infection, especially in individuals who cannot generate a humoral immune response to a prophylactic vaccination or natural infection. Individuals with inherited B-cell deficiency and cancer patients with treatment- or disease-linked B cell reduction fall into this category. T cells are crucial for COVID-19 outcomes and SARS-CoV-2 immunity maintenance in addition to B cell-driven humoral immunity.
CoVac-1, a peptide-based T-cell activator comprising of SARS-CoV-2 T-cell epitopes obtained from several viral proteins, coupled with the toll-like receptor 1 and 2 (TLR 1/2) agonist XS151, had a favorable efficacy and safety profile in a phase 1 trial among healthy adults considering the induction of COVID-19-specific T-cell responses, according to the authors of the current study. This T cell response was superior to those induced by COVID-19 or currently approved SARS-CoV-2 vaccines.
CoVac-1 comprises various SARS-CoV-2 human leukocyte antigen (HLA)-DR T cell epitopes generated from distinct SARS-CoV-2 proteins, such as nucleocapsid (N), spike (S), membrane (M), envelope (E), open reading frame 8 (ORF 8). Hence, CoVac-1 stimulates T-cell immunity autonomous of current SARS-CoV-2 variants of concern (VOCs).
About the study
In the current study, the researchers ran a Phase 1/2 open-label CoVac-1 experiment enrolling 54 individuals with acquired or congenital B-cell deficiency who received a single subcutaneous CoVac-1 dose in Germany. The study's primary aim was immunogenicity in terms of CoVac-1-triggered T-cell responses till day 28; the secondary objective was safety till day 56. In addition to B-cell deficiency, half of the patients had CD4 + T cell counts of less than 500/µl, highlighting the trial population's severe immunodeficiency.
The team evaluated the data on adverse events from the patients. T-cell reactions toward the six SARS-CoV-2 HLA-DR CoVac-1 T cell epitopes were measured utilizing enzyme-linked immunosorbent spot (ELISPOT) evaluations to determine immunogenicity. T-cell responses were measured in all eligible subjects at baseline/day 1, day 7, 14, and 28 following receiving CoVac-1.
Results
The study results showed that 94 patients with acquired or congenital B-cell deficiency were screened at three research sites in Germany from 6 July 2021 to 13 January 2022. In addition, CoVac-1 was given to 54 patients, 14 in the Phase 1 safety run-in and 40 in the Phase 2 portion of the experiment.
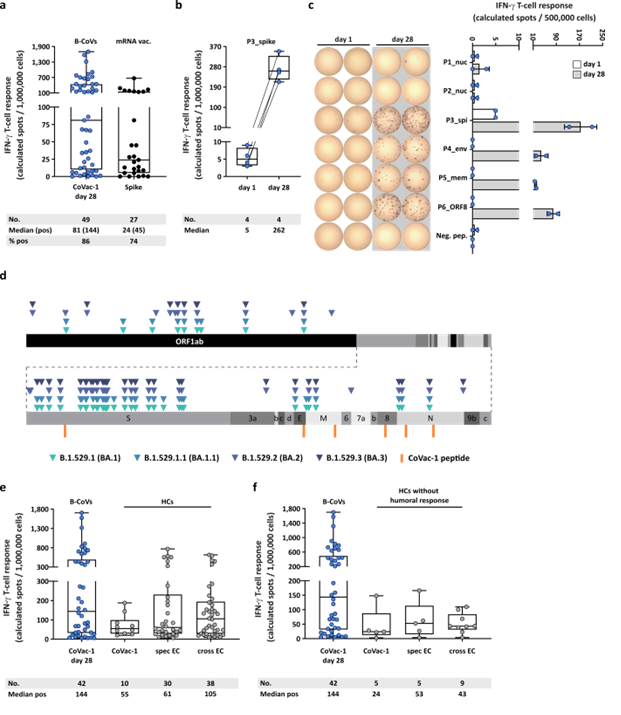
CoVac-1-induced T-cell responses with regard to Omicron variants and compared to mRNA vaccine- or infection-induced T-cell response. (a) CoVac-1-specific T-cell responses assessed ex vivo in study patients (day 28) compared to spike-specific T-cell responses prior to CoVac-1 administration in patients after second or third vaccination with approved mRNA vaccines (n = as indicated). (b) Intensities of P3_spike-induced IFN‑g T-cell responses assessed ex vivo in study patients (n = as indicated) prior to and on day 28 after CoVac-1 administration. (c) Exemplary ex vivo ELISPOT assays of one study patient (UPN12), with pre-existing T-cell responses to P3_spike, for the six CoVac-1 peptides on day 1 (white) and day 28 (grey). The intensities of IFN‑g T-cell responses are depicted as calculated spot counts (mean spot count of technical replicates minus the respective negative control). (d) Color-coded mutations described for SARS-CoV-2 Omicron variants are shown together with CoVac-1 peptides (orange). Positive T-cell responses to specific (spec) and cross-reactive (cross) T-cell epitope compositions (ECs) in (e) immunocompetent HCs (CoVac-1, spec EC, cross EC, n = as indicated)10,11 and (f) immunocompetent HCs without anti-SARS-CoV-2-antibody response after infection (CoVac-1, spec EC cross EC, n = as indicated) compared to positive IFN-g T-cell responses in study patients assessed ex vivo (B-CoVs, n = as indicated, day 28). (a,b,e,f) The intensity of IFN‑g T-cell responses is depicted as calculated spot counts (mean spot count of technical replicates minus the respective negative control). Box plots or combined box-line plots show median with 25th or 75th percentiles, and min/max whiskers. (c) Bars with mean, SD and single data points. no., number; EC, epitope composition; HCs, healthy COVID-19 convalescents; ORF, open reading frame; pos, positive.
Details on unsolicited and solicited adverse events were available for every participant via diary cards for 28 days following CoVac-1 administration and safety checkups till day 56. No subject dropped out of the study due to side effects. The authors did not observe CoVac-1-associated grade 4 or significant side effects. The anticipated formation of local granuloma was seen in 94% of study participants. On the other hand, systemic reactogenicity was mainly nonexistent or mild.
On day 28, SARS-CoV-2-selective T cell reactions were generated in 86% of the subjects and oriented to numerous CoVac-1 peptides, with an average of four out of six peptides identified by the patients' T cells. These responses were not influenced by any current SARS-CoV-2 Omicron mutants and were regulated by multipurpose T-helper 1 (Th1) CD4+ T cells. These Th1 cells had positivity for interferon γ (IFN-γ), CD107a, interleukin-2 (IL-2), and tumor necrosis factor (TNF).
According to subgroup analysis, on day 28, those with acquired B-cell deficit had superior response rates and percentages of CoVac-1-triggered T cells than individuals with congenital B-cell deficiency. There was no discernible variance in the frequency and strength of CoVac-1-generated T-cell responses among cancer patients receiving anti-CD20 treatment and those who did not.
Further, in B-cell lacking individuals and immunocompetent seroconverted/non-seroconverted COVID-19 recovered subjects, CoVac-1-generated T-cell responses outperformed S-specific T-cell responses following vaccination with messenger ribonucleic acid (mRNA) vaccines. CoVac-1 was also able to enhance pre-existing S-specific T-cell responses.
None of the participants demonstrated any humoral immune response to SARS-CoV-2 at research inclusion, despite receiving more than two doses of approved COVID-19 vaccines. However, low-degree SARS-CoV-2 anti-S immunoglobulin G (IgG) antibodies generation was noticed in patients upon a single CoVac-1 dose administration, despite continually negative results in serial SARS-CoV-2 polymerase chain reactions (PCRs).
Conclusions
The present study reported the immunogenicity, reactogenicity, and safety of CoVac-1 in the at-risk cohorts with acquired or congenital B-cell deficiency. Even in this drastically immunocompromised sample population, this investigation verified the excellent safety record and demonstrated robust de novo activation of T-cell responses following a single CoVac-1 dose.
The authors concluded that with an outstanding safety characteristic, CoVac-1 elicits broad and robust T-cell responses in individuals with antibody/B cell deficit, irrespective of existing SARS-CoV-2 VOCs. The current data support moving forward of CoVac-1 to a pivotal Phase 2/3 effectiveness and safety study. A long-term Phase 2/3 efficacy research using CoVac-1 is now being prepared to determine which phenotypes and frequencies of T cells are needed to help tackle COVID-19.
*Important notice
Preprints with Research Square publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Juliane Walz, Jonas Heitmann, Claudia Tandler et al. Phase I/II trial of a peptide-based COVID-19 T-cell activator in patients with B-cell deficiency, 02 June 2022, PREPRINT (Version 1) available at Research Square, https://doi.org/10.21203/rs.3.rs-1693355/v1, https://www.researchsquare.com/article/rs-1693355/v1
Posted in: Drug Trial News | Medical Research News | Disease/Infection News
Tags: Antibodies, Antibody, Antigen, B Cell, Cancer, CD4, Cell, Coronavirus, Coronavirus Disease COVID-19, covid-19, Efficacy, Enzyme, Frequency, Human Leukocyte Antigen, Immune Response, immunity, Immunodeficiency, Immunoglobulin, Interferon, Interleukin, Interleukin-2, Leukocyte, Membrane, Necrosis, Omicron, Pandemic, Peptides, Polymerase, Receptor, Research, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, T-Cell, Tumor, Tumor Necrosis Factor

Written by
Shanet Susan Alex
Shanet Susan Alex, a medical writer, based in Kerala, India, is a Doctor of Pharmacy graduate from Kerala University of Health Sciences. Her academic background is in clinical pharmacy and research, and she is passionate about medical writing. Shanet has published papers in the International Journal of Medical Science and Current Research (IJMSCR), the International Journal of Pharmacy (IJP), and the International Journal of Medical Science and Applied Research (IJMSAR). Apart from work, she enjoys listening to music and watching movies.
Source: Read Full Article