Natural infection or vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) confer considerable protection against reinfection and reduce the risk of severe Coronavirus disease 2019 (COVID-19) outcomes.
However, reports on decaying neutralizing antibody (Ab) levels against SARS-CoV-2 have raised questions regarding long-term immunity. Sporadic breakthrough infections have been reported after vaccination due to lower antibody levels to SARS-CoV-2 spike protein, prompting consideration of booster doses. Therefore, urgent studies are needed to identify factors associated with protection to assist in the future deployment of vaccines.
Diana Zhong and colleagues from Johns Hopkins University have conducted a cohort study on healthcare workers (HWs) to probe SARS-CoV-2 spike Immunoglobulin G (IgG) Ab levels, comparing their decay in vaccinated individuals with and without prior SARS-CoV-2 infection.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer view.
Cohort of healthcare workers
The HWs included in the study had either a serum sample collected post-SARS-CoV-2 infection (defined as positive SARS-CoV-2 PCR) (n=98), and/or a serum sample collected ≥ 14 days after the second dose of an mRNA SARS-CoV-2 vaccine (n=1,960).
Enzyme-linked immunosorbent assay (ELISA) was used to quantify Abs in the serum and linear regression models, adjusting for vaccine type, age, and sex, were used to compare post-vaccination Ab levels.
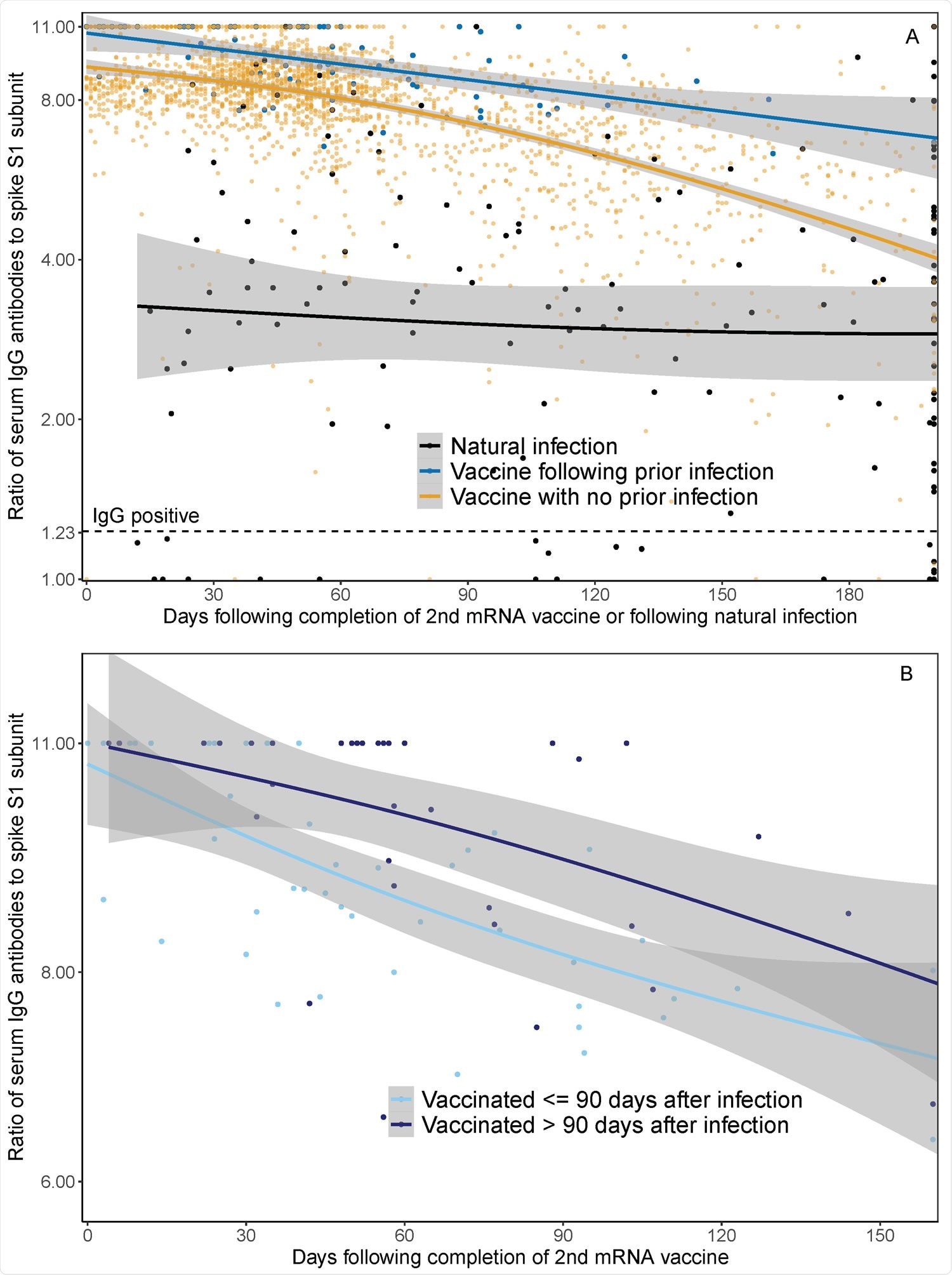
Major findings
- Serum spike IgG Ab levels were higher after vaccination than after natural infection.
- Individuals with prior infection before vaccination continued to have higher serum IgG Ab levels, at 1, 3, and 6 months (following completion of 2nd dose of vaccine, or following natural infection), when compared to individuals with infection alone or those with vaccination alone.
- Individuals with PCR confirmed infection > 90 days before vaccination had higher post-vaccination Ab levels than individuals infected ≤ 90 days before vaccination by approximately 10 percent, as studied at 1 and 3 months.
Longer interval between infection and first vaccine dose enhances Ab response
In agreement with another study comparing extended vaccine dosing intervals, the authors suggest a longer interval of at least 90 days between infection and first vaccine dose (second exposure) for enhanced Ab response. Subsequently, the booster dose which provides a third exposure induces a more durable Ab response.
"Vaccination after natural infection reduces risk of reinfection," the team concludes.
The researchers also highlight the need to inquire whether the improved post-vaccination Ab durability in prior infected individuals is attributable to the number of exposures, the interval between exposures, or a complex interplay between natural and vaccine-derived immunity.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Zhong D., et al. (2021). Impact of prior SARS-CoV-2 infection on post-vaccination SARS-CoV-2 spike IgG antibodies in a longitudinal cohort of healthcare workers. medRxiv preprint server. doi: https://doi.org/10.1101/2021.09.16.21263576, https://www.medrxiv.org/content/10.1101/2021.09.16.21263576v1
Posted in: Medical Research News | Disease/Infection News | Healthcare News
Tags: Antibodies, Antibody, Assay, Coronavirus, Coronavirus Disease COVID-19, Enzyme, Healthcare, immunity, Immunoglobulin, Protein, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Vaccine

Written by
Namita Mitra
After earning a bachelor’s degree in Veterinary Sciences and Animal Health (BVSc) in 2013, Namita went on to pursue a Master of Veterinary Microbiology from GADVASU, India. Her Master’s research on the molecular and histopathological diagnosis of avian oncogenic viruses in poultry brought her two national awards. In 2013, she was conferred a doctoral degree in Animal Biotechnology that concluded with her research findings on expression profiling of apoptosis-associated genes in canine mammary tumors. Right after her graduation, Namita worked as Assistant Professor of Animal Biotechnology and taught the courses of Animal Cell Culture, Animal Genetic Engineering, and Molecular Immunology.
Source: Read Full Article
